Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0T1FJ
|
||||
| Former ID |
DNC014138
|
||||
| Drug Name |
4-(Aminomethyl)-7-(benzyloxy)-2H-chromen-2-one
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Discovery agent | Investigative | [530434] | ||
| Structure |
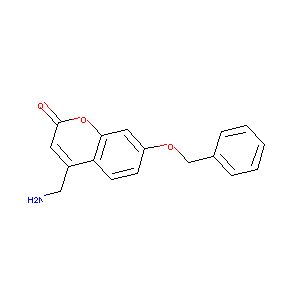
|
Download2D MOL |
|||
| Formula |
C17H15NO3
|
||||
| Canonical SMILES |
C1=CC=C(C=C1)COC2=CC3=C(C=C2)C(=CC(=O)O3)CN
|
||||
| InChI |
1S/C17H15NO3/c18-10-13-8-17(19)21-16-9-14(6-7-15(13)16)20-11-12-4-2-1-3-5-12/h1-9H,10-11,18H2
|
||||
| InChIKey |
XCWHFSADYQMGEA-UHFFFAOYSA-N
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Amine oxidase [flavin-containing] A | Target Info | Inhibitor | [530434] | |
| Amine oxidase [flavin-containing] B | Target Info | Inhibitor | [530434] | ||
| BioCyc Pathway | Superpathway of tryptophan utilization | ||||
| Dopamine degradation | |||||
| Putrescine degradation III | |||||
| Noradrenaline and adrenaline degradation | |||||
| Serotonin degradation | |||||
| Superpathway of melatonin degradation | |||||
| Melatonin degradation IIPWY66-401:Superpathway of tryptophan utilization | |||||
| Tryptophan degradation via tryptamine | |||||
| KEGG Pathway | Glycine, serine and threonine metabolism | ||||
| Arginine and proline metabolism | |||||
| Histidine metabolism | |||||
| Tyrosine metabolism | |||||
| Phenylalanine metabolism | |||||
| Tryptophan metabolism | |||||
| Drug metabolism - cytochrome P450 | |||||
| Metabolic pathways | |||||
| Serotonergic synapse | |||||
| Dopaminergic synapse | |||||
| Cocaine addiction | |||||
| Amphetamine addiction | |||||
| Alcoholismhsa00260:Glycine, serine and threonine metabolism | |||||
| Alcoholism | |||||
| NetPath Pathway | IL4 Signaling Pathway | ||||
| Pathway Interaction Database | Alpha-synuclein signaling | ||||
| References | |||||
| Ref 530434 | J Med Chem. 2009 Nov 12;52(21):6685-706.Discovery of a novel class of potent coumarin monoamine oxidase B inhibitors: development and biopharmacological profiling of 7-[(3-chlorobenzyl)oxy]-4-[(methylamino)methyl]-2H-chromen-2-one methanesulfonate (NW-1772) as a highly potent, selective, reversible, and orally active monoamine oxidase B inhibitor. | ||||
| Ref 530434 | J Med Chem. 2009 Nov 12;52(21):6685-706.Discovery of a novel class of potent coumarin monoamine oxidase B inhibitors: development and biopharmacological profiling of 7-[(3-chlorobenzyl)oxy]-4-[(methylamino)methyl]-2H-chromen-2-one methanesulfonate (NW-1772) as a highly potent, selective, reversible, and orally active monoamine oxidase B inhibitor. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

