Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0Y5ZT
|
||||
| Former ID |
DIB013290
|
||||
| Drug Name |
KRP-203
|
||||
| Synonyms |
KNF-299; KRP-203-P prodrug, Kyorin
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Cutaneous lupus erythematosus [ICD9: 710; ICD10:M32] | Phase 2 | [523356] | ||
| Company |
Kyorin Pharmaceutical Co Ltd
|
||||
| Structure |
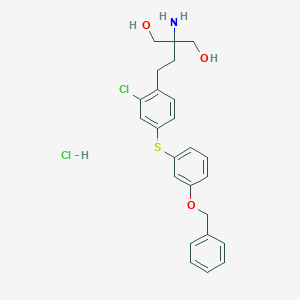
|
Download2D MOL |
|||
| Formula |
C24H26ClNO3S
|
||||
| Canonical SMILES |
NC(CO)(CCc1ccc(Sc2cccc(OCc3ccccc3)c2)cc1Cl)CO.Cl
|
||||
| CAS Number |
CAS 509088-69-1
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Sphingosine 1-phosphate receptor 1 | Target Info | Agonist | [532341], [532456] | |
| References | |||||
| Ref 532341 | KRP-203, sphingosine 1-phosphate receptor type 1 agonist, ameliorates atherosclerosis in LDL-R-/- mice. Arterioscler Thromb Vasc Biol. 2013 Jul;33(7):1505-12. | ||||
| Ref 532456 | Targeting the sphingosine-1-phosphate axis in cancer, inflammation and beyond. Nat Rev Drug Discov. 2013 Sep;12(9):688-702. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

