Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D07RUV
|
||||
| Former ID |
DIB000959
|
||||
| Drug Name |
Terflavoxate
|
||||
| Synonyms |
ND-907; Rec-15-2053
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Urinary incontinence [ICD9: 788.3; ICD10:N39.3, N39.4, R32] | Discontinued in Phase 2 | [545426] | ||
| Company |
Recordati SpA
|
||||
| Structure |
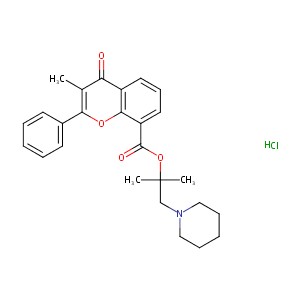
|
Download2D MOL |
|||
| Formula |
C26H29NO4
|
||||
| Canonical SMILES |
c12oc(c(c(=O)c2cccc1C(=O)OC(CN1CCCCC1)(C)C)C)c1ccccc1.C<br />l
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Muscarinic receptor | Target Info | Modulator | [534003] | |
| PathWhiz Pathway | Muscle/Heart Contraction | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

