Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0N2GQ
|
||||
| Former ID |
DIB007332
|
||||
| Drug Name |
ATI-0917
|
||||
| Synonyms |
FB-636
|
||||
| Indication | Human immunodeficiency virus infection [ICD9: 279.3; ICD10:B20-B26] | Phase 1 | [530051] | ||
| Company |
The Procter & Gamble Co
|
||||
| Structure |
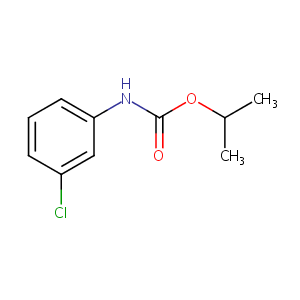
|
Download2D MOL |
|||
| Canonical SMILES |
c1(NC(=O)OC(C)C)cc(ccc1)Cl
|
||||
| CAS Number |
CAS 101-21-3
|
||||
| Target and Pathway | |||||
| Target(s) | HIV REV protein | Target Info | Inhibitor | [530051] | |
| Reactome | Uncoating of the HIV Virion | ||||
| Budding and maturation of HIV virion | |||||
| Integration of provirus | |||||
| Early Phase of HIV Life Cycle | |||||
| Minus-strand DNA synthesis | |||||
| Plus-strand DNA synthesis | |||||
| 2-LTR circle formation | |||||
| Binding and entry of HIV virion | |||||
| Assembly Of The HIV Virion | |||||
| Integration of viral DNA into host genomic DNA | |||||
| Autointegration results in viral DNA circles | |||||
| APOBEC3G mediated resistance to HIV-1 infection | |||||
| Vpr-mediated nuclear import of PICs | |||||
| WikiPathways | Host Interactions of HIV factors | ||||
| HIV Life Cycle | |||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

