Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D05ADP
|
|||
| Former ID |
DCL001080
|
|||
| Drug Name |
R-roscovitine
|
|||
| Synonyms |
Seliciclib; roscovitine; 186692-46-6; R-Roscovitine; (R)-roscovitine; CYC202; CYC-202; CYC 202; 2-(R)-(1-Ethyl-2-hydroxyethylamino)-6-benzylamino-9-isopropylpurine; UNII-0ES1C2KQ94; Roscovitine (Seliciclib,CYC202); NSC 701554; AL-39256; Roscovitine; CHEMBL14762; 0ES1C2KQ94; CHEBI:45307; NSC701554; NSC-701554; (2R)-2-[[6-(benzylamino)-9-isopropyl-purin-2-yl]amino]butan-1-ol; (R)-2-((6-(Benzylamino)-9-isopropyl-9H-purin-2-yl)amino)butan-1-ol; (2R)-2-[[6-(benzylamino)-9-propan-2-ylpurin-2-yl]amino]butan-1-ol; RRC; Rosco; M02443; BMK1-E12; CYC202, Seliciclib, R-roscovitine, Roscovitine; (2r)-2-{[6-(benzylamino)-9-isopropyl-9h-purin-2-yl]amino}-1-butanol; 2-(R)-[[9-(1-Methylethyl)-6-[(phenylmethyl)amino]-9H-purin-2-yl]amino]-1-butanol; 2-[[9-(1-Methylethyl)-6-[(phenylmethyl)amino]-9H-purin-2-yl]amino]-(R)-1-butanol; 6-(Benzylamino)-2(R)-[[1-(hydroxymethyl)propyl]amino]-9-isopropylpurine
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Non-small-cell lung cancer [ICD-11: 2C25.Y] | Phase 2 | [1], [2] | |
| Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C76-C80; ICD-9: 140-229] | Phase 2 | [3] | ||
| Nasopharyngeal carcinoma [ICD-11: 2B6B; ICD-9: 147] | Investigative | [4], [5] | ||
| Company |
Cyclacel
|
|||
| Structure |
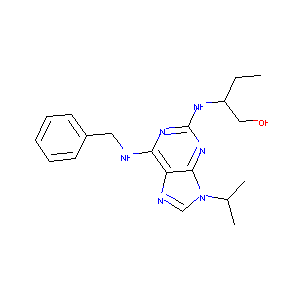 |
Download2D MOL |
||
| Formula |
C19H26N6O
|
|||
| Canonical SMILES |
CCC(CO)NC1=NC(=C2C(=N1)N(C=N2)C(C)C)NCC3=CC=CC=C3
|
|||
| InChI |
1S/C19H26N6O/c1-4-15(11-26)22-19-23-17(20-10-14-8-6-5-7-9-14)16-18(24-19)25(12-21-16)13(2)3/h5-9,12-13,15,26H,4,10-11H2,1-3H3,(H2,20,22,23,24)/t15-/m1/s1
|
|||
| InChIKey |
BTIHMVBBUGXLCJ-OAHLLOKOSA-N
|
|||
| CAS Number |
CAS 186692-46-6
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
527377, 584365, 610560, 6436229, 7890318, 10253958, 11108321, 11120183, 11120671, 11121159, 11121724, 11122204, 11147266, 11362832, 11365394, 11367956, 11370895, 11370896, 11373557, 11376118, 11440772, 12015583, 14719432, 14720319, 14778719, 14876445, 24278682, 26753649, 26753650, 26759456, 26759458, 46235405, 46393775, 47364935, 47364936, 47439996, 47736219, 47736220, 48034852, 48034853, 48184761, 50066372, 50087155, 50100113, 50107006, 50107007, 53789190, 57349053, 68533699, 80722361
|
|||
| ChEBI ID |
CHEBI:45307
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6035). | |||
| REF 2 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800010117) | |||
| REF 3 | ClinicalTrials.gov (NCT02160730) Treatment of Cushing's Disease With R-roscovitine. U.S. National Institutes of Health. | |||
| REF 4 | Therapeutic efficacy of seliciclib in combination with ionizing radiation for human nasopharyngeal carcinoma.Clin Cancer Res. 2009 Jun 1;15(11):3716-24. | |||
| REF 5 | Pharmacodynamic effects of seliciclib, an orally administered cell cycle modulator, in undifferentiated nasopharyngeal cancer.Clin Cancer Res. 2009 Feb 15;15(4):1435-42. | |||
| REF 6 | Cell cycle kinases as therapeutic targets for cancer. Nat Rev Drug Discov. 2009 Jul;8(7):547-66. | |||
| REF 7 | A comparison of physicochemical property profiles of marketed oral drugs and orally bioavailable anti-cancer protein kinase inhibitors in clinical development. Curr Top Med Chem. 2007;7(14):1408-22. | |||
| REF 8 | Cdk2 plays a critical role in hepatocyte cell cycle progression and survival in the setting of cyclin D1 expression in vivo. Cell Cycle. 2009 Sep 1;8(17):2802-9. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

