Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D00LFB
|
|||
| Former ID |
DAP000937
|
|||
| Drug Name |
Ritodrine
|
|||
| Synonyms |
Prepar; Ritodrina; Ritodrinium; Utopar; Yutopar; RITODRINE HCL; DU-21220; Ritodrina [INN-Spanish]; Ritodrinium [INN-Latin]; Yutopar (TN); Yutopar S.R; Ritodrine (USAN/INN); Ritodrine [USAN:BAN:INN]; P-Hydroxy-alpha-(1-((p-hydroxyphenethyl)amino)ethyl)benzyl alcohol; Erythro-p-Hydroxy-alpha-(1-((p-hydroxyphenethyl)amino)ethyl)benzyl alcohol; 2-(4-Hydroxyphenethylamino)-1-(4-hydroxyphenyl)propanol; 4-[2-[[(1R,2R)-1-hydroxy-1-(4-hydroxyphenyl)propan-2-yl]amino]ethyl]phenol; 4-[2-[[(1R,2S)-1-hydroxy-1-(4-hydroxyphenyl)propan-2-yl]amino]ethyl]phenol; 4-[2-[[(1S,2R)-1-hydroxy-1-(4-hydroxyphenyl)propan-2-yl]amino]ethyl]phenol; 4-[2-[[(1S,2S)-1-hydroxy-1-(4-hydroxyphenyl)propan-2-yl]amino]ethyl]phenol; 4-[2-[[1-hydroxy-1-(4-hydroxyphenyl)propan-2-yl]amino]ethyl]phenol
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Premature labour [ICD-11: JB00; ICD-10: O60] | Approved | [1], [2] | |
| Therapeutic Class |
Sympathomimetics
|
|||
| Structure |
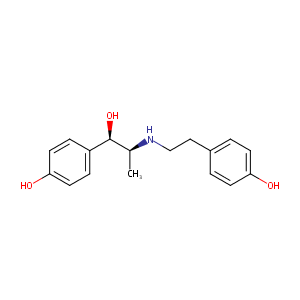 |
Download2D MOL |
||
| Formula |
C17H21NO3
|
|||
| Canonical SMILES |
CC(C(C1=CC=C(C=C1)O)O)NCCC2=CC=C(C=C2)O
|
|||
| InChI |
1S/C17H21NO3/c1-12(17(21)14-4-8-16(20)9-5-14)18-11-10-13-2-6-15(19)7-3-13/h2-9,12,17-21H,10-11H2,1H3/t12-,17-/m0/s1
|
|||
| InChIKey |
IOVGROKTTNBUGK-SJCJKPOMSA-N
|
|||
| CAS Number |
CAS 26652-09-5
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
9448, 7849418, 10782682, 11466377, 11467497, 11486074, 14922364, 34675370, 46505273, 47365457, 47515589, 47736756, 47885654, 49698446, 49880716, 50124284, 57311534, 77543448, 85788060, 103207155, 104316379, 117557196, 134223139, 134338212, 134997132, 135841429, 137001406, 139650986, 160964208, 163358501, 164038406, 178103867, 179150198, 184546165, 198973441, 223440870, 225144308, 226420705
|
|||
| ChEBI ID |
CHEBI:8872
|
|||
| SuperDrug ATC ID |
G02CA01
|
|||
| SuperDrug CAS ID |
cas=026652095
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Adrenergic receptor beta-2 (ADRB2) | Target Info | Modulator | [3] |
| KEGG Pathway | Calcium signaling pathway | |||
| cGMP-PKG signaling pathway | ||||
| cAMP signaling pathway | ||||
| Neuroactive ligand-receptor interaction | ||||
| Endocytosis | ||||
| Adrenergic signaling in cardiomyocytes | ||||
| Salivary secretion | ||||
| NetPath Pathway | TCR Signaling Pathway | |||
| Panther Pathway | Heterotrimeric G-protein signaling pathway-Gi alpha and Gs alpha mediated pathway | |||
| Beta2 adrenergic receptor signaling pathway | ||||
| Pathway Interaction Database | Arf6 trafficking events | |||
| Arf6 signaling events | ||||
| Reactome | Adrenoceptors | |||
| G alpha (s) signalling events | ||||
| WikiPathways | Monoamine GPCRs | |||
| Calcium Regulation in the Cardiac Cell | ||||
| GPCRs, Class A Rhodopsin-like | ||||
| Vitamin D Receptor Pathway | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| GPCRs, Other | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7294). | |||
| REF 2 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 071438. | |||
| REF 3 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

