Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D02ZJI
|
|||
| Former ID |
DNC000873
|
|||
| Drug Name |
Levalbuterol
|
|||
| Synonyms |
Levosalbutamol; 34391-04-3; (R)-salbutamol; Xopenex; R-Salbutamol; R-Albuterol; (r)-(-)-salbutamol; UNII-EDN2NBH5SS; 4-[(1R)-2-(tert-butylamino)-1-hydroxyethyl]-2-(hydroxymethyl)phenol; EDN2NBH5SS; CHEBI:8746; Levosalbutamol (INN); Levosalbutamol [INN]; (R)-albuterol; m-Xylene-alpha,alpha'-diol, alpha(sup 1)-((tert-butylamino)methyl)-4-hydroxy-, (R)-(-)-; (-)-alpha(sup 1)-(((1,1-Dimethylethyl)amino)methyl)-4-hydroxy-1,3-benzenedimethanol
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Asthma [ICD-11: CA23; ICD-10: J45, J45.8; ICD-9: 493] | Approved | [1], [2] | |
| Structure |
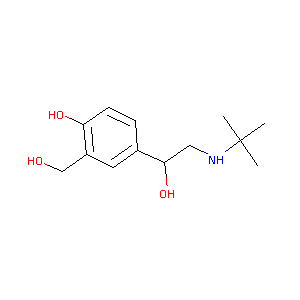 |
Download2D MOL |
||
| Formula |
C13H21NO3
|
|||
| Canonical SMILES |
CC(C)(C)NCC(C1=CC(=C(C=C1)O)CO)O
|
|||
| InChI |
1S/C13H21NO3/c1-13(2,3)14-7-12(17)9-4-5-11(16)10(6-9)8-15/h4-6,12,14-17H,7-8H2,1-3H3/t12-/m0/s1
|
|||
| InChIKey |
NDAUXUAQIAJITI-LBPRGKRZSA-N
|
|||
| CAS Number |
CAS 34391-04-3
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
13934, 10240794, 10852016, 11111805, 11111806, 11112645, 11113612, 14798337, 26744252, 29303834, 49879812, 50071332, 57340474, 77876163, 93166666, 96024814, 99036159, 99234398, 99774850, 103266935, 103911314, 104418876, 117814444, 124881447, 125325478, 126524239, 126670038, 128684762, 131311264, 131835145, 135078939, 135376066, 136965309, 137001318, 139121247, 164795671, 175267044, 175269554, 177748808, 179150348, 184546277, 223519271, 223668238, 223982365, 226396078, 252091683, 252166097
|
|||
| ChEBI ID |
CHEBI:8746
|
|||
| ADReCS Drug ID | BADD_D01262 ; BADD_D01263 ; BADD_D01264 | |||
| SuperDrug ATC ID |
R03AC02
|
|||
| SuperDrug CAS ID |
cas=018559949
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Adrenergic receptor beta-2 (ADRB2) | Target Info | Antagonist | [3] |
| KEGG Pathway | Calcium signaling pathway | |||
| cGMP-PKG signaling pathway | ||||
| cAMP signaling pathway | ||||
| Neuroactive ligand-receptor interaction | ||||
| Endocytosis | ||||
| Adrenergic signaling in cardiomyocytes | ||||
| Salivary secretion | ||||
| NetPath Pathway | TCR Signaling Pathway | |||
| Panther Pathway | Heterotrimeric G-protein signaling pathway-Gi alpha and Gs alpha mediated pathway | |||
| Beta2 adrenergic receptor signaling pathway | ||||
| Pathway Interaction Database | Arf6 trafficking events | |||
| Arf6 signaling events | ||||
| Reactome | Adrenoceptors | |||
| G alpha (s) signalling events | ||||
| WikiPathways | Monoamine GPCRs | |||
| Calcium Regulation in the Cardiac Cell | ||||
| GPCRs, Class A Rhodopsin-like | ||||
| Vitamin D Receptor Pathway | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| GPCRs, Other | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 2 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800005434) | |||
| REF 3 | Beta-blockers in the treatment of hypertension: are there clinically relevant differences Postgrad Med. 2009 May;121(3):90-8. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

