Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0B3MX
|
|||
| Former ID |
DIB000632
|
|||
| Drug Name |
NBI-6024
|
|||
| Synonyms |
Diabetes therapeutics, Neurocrine/Taisho; APL (diabetes), Neurocrine/Taisho; Applied Peptide Ligand(diabetes), Neurocrine/Taisho; Insulin B9-23 peptide analog, Taisho/Neurocrine
Click to Show/Hide
|
|||
| Indication | Type-1 diabetes [ICD-11: 5A10; ICD-9: 250] | Discontinued in Phase 2 | [1] | |
| Company |
Neurocrine Biosciences
|
|||
| Structure |
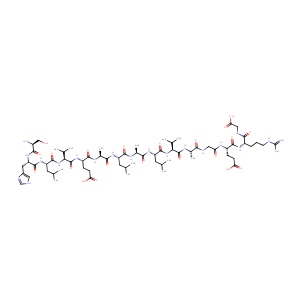 |
Download2D MOL |
||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800011773) | |||
| REF 2 | No effect of the altered peptide ligand NBI-6024 on beta-cell residual function and insulin needs in new-onset type 1 diabetes.Diabetes Care.2009 Nov;32(11):2036-40. | |||
| REF 3 | Immunological characterization and therapeutic activity of an altered-peptide ligand, NBI-6024, based on the immunodominant type 1 diabetes autoantigen insulin B-chain (9-23) peptide. Diabetes. 2002 Jul;51(7):2126-34. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

