Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T82841
(Former ID: TTDNC00576)
|
|||||
| Target Name |
Insulin (INS)
|
|||||
| Synonyms |
Insulin
Click to Show/Hide
|
|||||
| Gene Name |
INS
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 3 Target-related Diseases | + | ||||
| 1 | Acute diabete complication [ICD-11: 5A2Y] | |||||
| 2 | Kidney malfunction [ICD-11: MG02] | |||||
| 3 | Type-1/2 diabete [ICD-11: 5A10-5A11] | |||||
| Function |
Insulin decreases blood glucose concentration. It increases cell permeability to monosaccharides, amino acids and fatty acids. It accelerates glycolysis, the pentose phosphate cycle, and glycogen synthesis in liver.
Click to Show/Hide
|
|||||
| UniProt ID | ||||||
| Sequence |
MALWMRLLPLLALLALWGPDPAAAFVNQHLCGSHLVEALYLVCGERGFFYTPKTRREAED
LQVGQVELGGGPGAGSLQPLALEGSLQKRGIVEQCCTSICSLYQLENYCN Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | PDB | ||||
| ADReCS ID | BADD_A02189 ; BADD_A02399 ; BADD_A02651 | |||||
| HIT2.0 ID | T89L4V | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 9 Approved Drugs | + | ||||
| 1 | Insulin degludec | Drug Info | Approved | Type-1/2 diabetes | [2] | |
| 2 | Insulin determir | Drug Info | Approved | Diabetic complication | [3] | |
| 3 | Insulin Susp Isophane Recombinant Human | Drug Info | Approved | Diabetic complication | [3] | |
| 4 | Insulin Susp Protamine Zinc Beef/Pork | Drug Info | Approved | Diabetic complication | [3] | |
| 5 | Insulin Zinc Susp Recombinant Human | Drug Info | Approved | Diabetic complication | [3] | |
| 6 | Insulin-aspart | Drug Info | Approved | Diabetic complication | [4] | |
| 7 | Insulin-detemir | Drug Info | Approved | Type-1/2 diabetes | [1], [4] | |
| 8 | Insulin-glargine | Drug Info | Approved | Diabetic complication | [4], [5] | |
| 9 | Inulin | Drug Info | Approved | Measure kidney function | [4] | |
| Clinical Trial Drug(s) | [+] 12 Clinical Trial Drugs | + | ||||
| 1 | LY2605541 | Drug Info | Phase 3 | Type-1/2 diabetes | [8] | |
| 2 | LY2963016 | Drug Info | Phase 3 | Type-1/2 diabetes | [9] | |
| 3 | SAR342434 | Drug Info | Phase 3 | Diabetic complication | [10] | |
| 4 | MER-3001 | Drug Info | Phase 2 | Type-1 diabetes | [11] | |
| 5 | NNC-0123-0000-0338 | Drug Info | Phase 2 | Diabetic complication | [12] | |
| 6 | SAR-161271 | Drug Info | Phase 1/2 | Type-1 diabetes | [13] | |
| 7 | Adjustable basal insulin | Drug Info | Phase 1 | Diabetic complication | [14] | |
| 8 | IBC-VS01 | Drug Info | Phase 1 | Type-1 diabetes | [15] | |
| 9 | NP-500 | Drug Info | Phase 1 | Type-2 diabetes | [16] | |
| 10 | OI287GT | Drug Info | Phase 1 | Type-1/2 diabetes | [17] | |
| 11 | OI362GT | Drug Info | Phase 1 | Type-1/2 diabetes | [18] | |
| 12 | SBS-1000 | Drug Info | Phase 1 | Diabetic complication | [19] | |
| Discontinued Drug(s) | [+] 4 Discontinued Drugs | + | ||||
| 1 | NBI-6024 | Drug Info | Discontinued in Phase 2 | Type-1 diabetes | [20] | |
| 2 | S-15261 | Drug Info | Discontinued in Phase 2 | Diabetic complication | [21] | |
| 3 | FT-105 | Drug Info | Discontinued in Phase 1 | Diabetic complication | [22] | |
| 4 | Oral insulin | Drug Info | Terminated | Type-1 diabetes | [23] | |
| Mode of Action | [+] 2 Modes of Action | + | ||||
| Modulator | [+] 25 Modulator drugs | + | ||||
| 1 | Insulin degludec | Drug Info | [2] | |||
| 2 | Insulin determir | Drug Info | [1] | |||
| 3 | Insulin Susp Isophane Recombinant Human | Drug Info | [24] | |||
| 4 | Insulin Susp Protamine Zinc Beef/Pork | Drug Info | [24] | |||
| 5 | Insulin Zinc Susp Recombinant Human | Drug Info | [24] | |||
| 6 | Insulin-aspart | Drug Info | [24] | |||
| 7 | Insulin-detemir | Drug Info | [24] | |||
| 8 | Insulin-glargine | Drug Info | [24] | |||
| 9 | Inulin | Drug Info | [24] | |||
| 10 | LY2605541 | Drug Info | [25] | |||
| 11 | SAR342434 | Drug Info | [27] | |||
| 12 | MER-3001 | Drug Info | [28] | |||
| 13 | NNC-0123-0000-0338 | Drug Info | [29] | |||
| 14 | SAR-161271 | Drug Info | [30] | |||
| 15 | Adjustable basal insulin | Drug Info | [31] | |||
| 16 | IBC-VS01 | Drug Info | [32] | |||
| 17 | NP-500 | Drug Info | [33] | |||
| 18 | OI287GT | Drug Info | [34] | |||
| 19 | OI362GT | Drug Info | [35] | |||
| 20 | SBS-1000 | Drug Info | [36] | |||
| 21 | NBI-6024 | Drug Info | [37], [38] | |||
| 22 | S-15261 | Drug Info | [39] | |||
| 23 | FT-105 | Drug Info | [40] | |||
| 24 | Oral insulin | Drug Info | [41] | |||
| 25 | Insulin molecules, Novo | Drug Info | [42] | |||
| Agonist | [+] 1 Agonist drugs | + | ||||
| 1 | LY2963016 | Drug Info | [26] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Urea | Ligand Info | |||||
| Structure Description | Structure of human insulin cocrystallized with ARG-12 peptide in presence of urea | PDB:2OMH | ||||
| Method | X-ray diffraction | Resolution | 1.36 Å | Mutation | No | [43] |
| PDB Sequence |
GIVEQCCTSI
10 CSLYQLENYC20 N
|
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target and Ligand Pair | ||||||
| Ligand Name: Arginine | Ligand Info | |||||
| Structure Description | Human insulin in complex with serotonin and arginine | PDB:5MT9 | ||||
| Method | X-ray diffraction | Resolution | 1.88 Å | Mutation | No | [44] |
| PDB Sequence |
HLCGSHLVEA
14 LYLVCGERGF24 FYTPK
|
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target and Ligand Pair | ||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| MAPK signaling pathway | hsa04010 | Affiliated Target |
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Ras signaling pathway | hsa04014 | Affiliated Target |
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Rap1 signaling pathway | hsa04015 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| cGMP-PKG signaling pathway | hsa04022 | Affiliated Target |
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| HIF-1 signaling pathway | hsa04066 | Affiliated Target |
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| FoxO signaling pathway | hsa04068 | Affiliated Target |
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Phospholipase D signaling pathway | hsa04072 | Affiliated Target |
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Oocyte meiosis | hsa04114 | Affiliated Target |

|
| Class: Cellular Processes => Cell growth and death | Pathway Hierarchy | ||
| Autophagy - animal | hsa04140 | Affiliated Target |
|
| Class: Cellular Processes => Transport and catabolism | Pathway Hierarchy | ||
| mTOR signaling pathway | hsa04150 | Affiliated Target |
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| PI3K-Akt signaling pathway | hsa04151 | Affiliated Target |
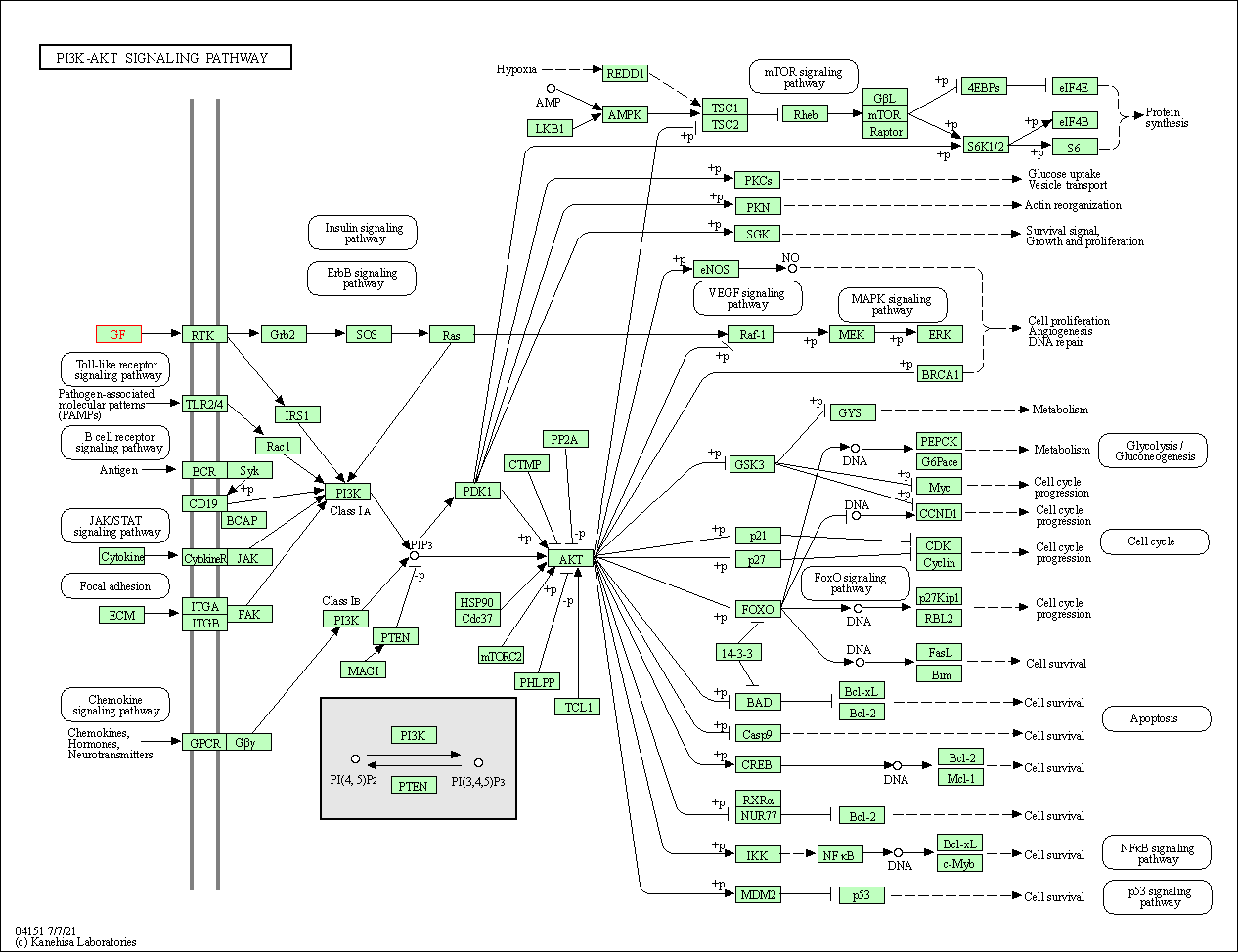
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| AMPK signaling pathway | hsa04152 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Longevity regulating pathway | hsa04211 | Affiliated Target |
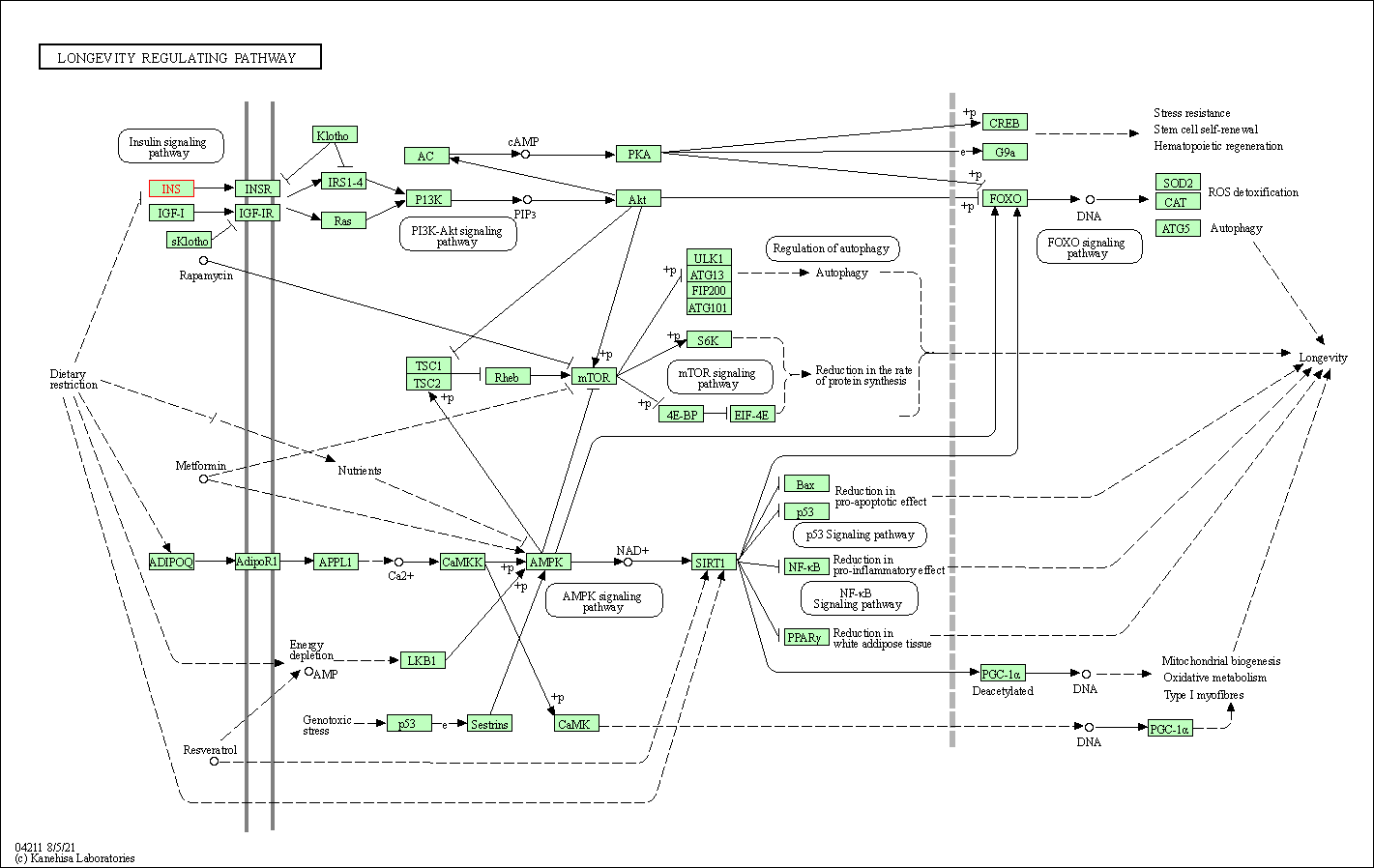
|
| Class: Organismal Systems => Aging | Pathway Hierarchy | ||
| Longevity regulating pathway - multiple species | hsa04213 | Affiliated Target |
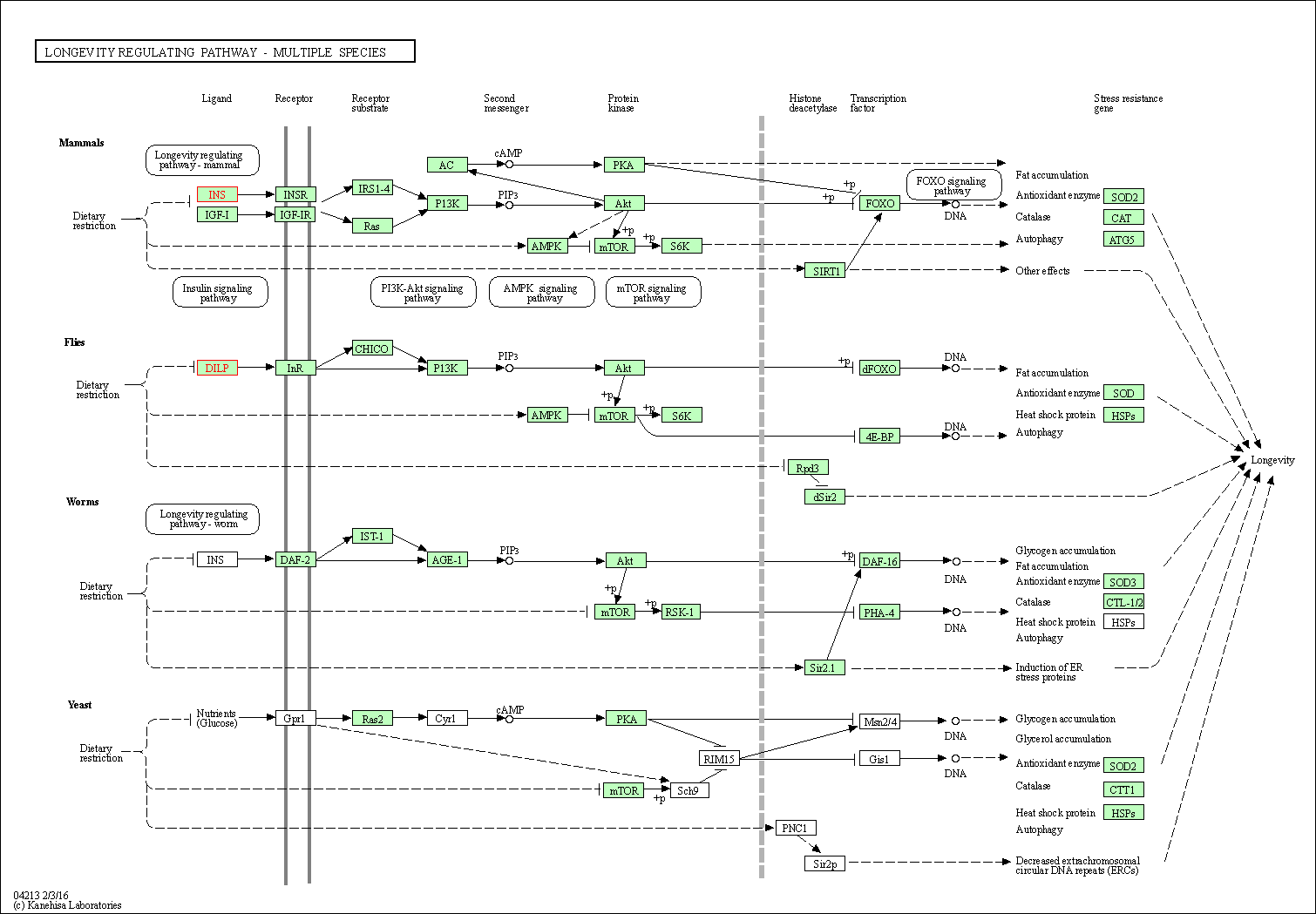
|
| Class: Organismal Systems => Aging | Pathway Hierarchy | ||
| Regulation of actin cytoskeleton | hsa04810 | Affiliated Target |
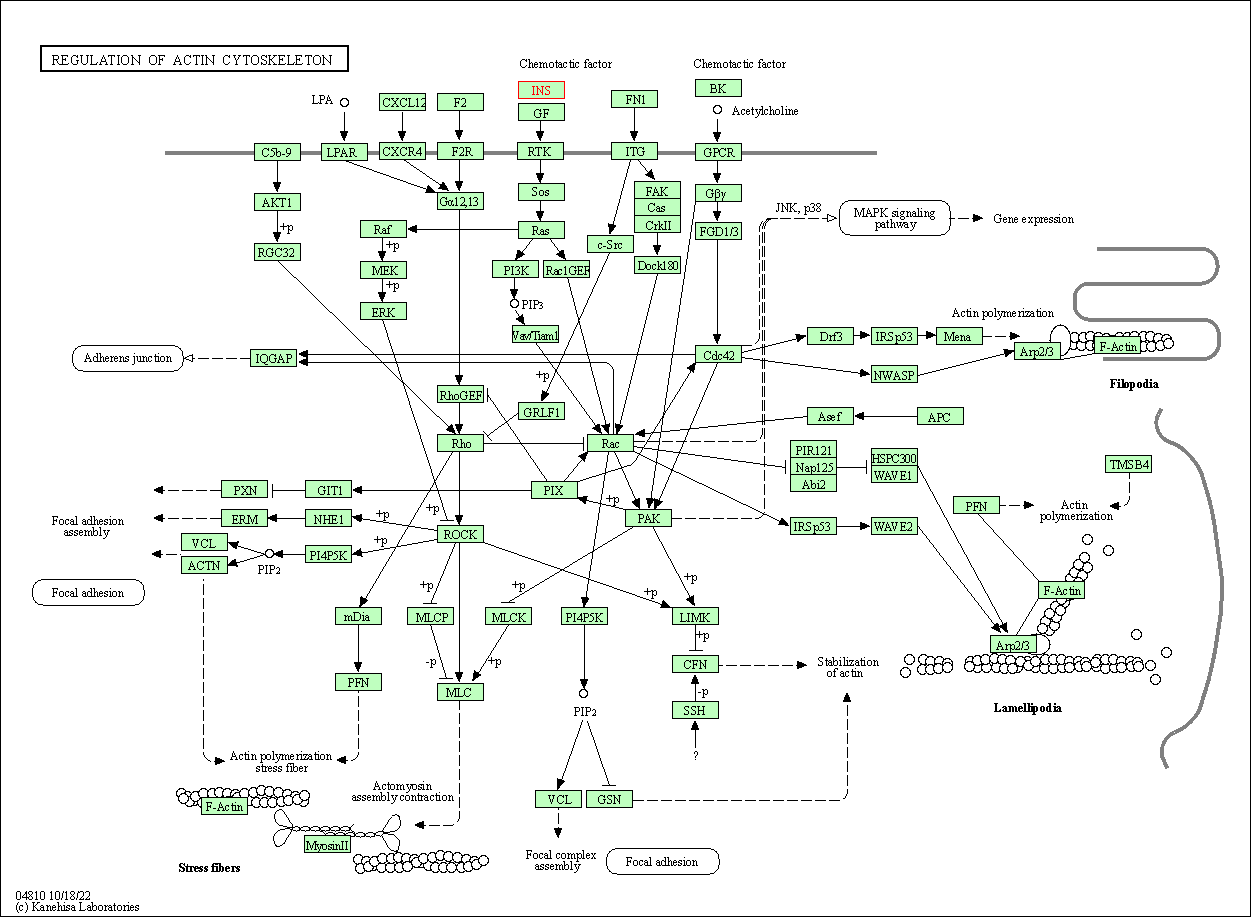
|
| Class: Cellular Processes => Cell motility | Pathway Hierarchy | ||
| Insulin signaling pathway | hsa04910 | Affiliated Target |
|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Insulin secretion | hsa04911 | Affiliated Target |
|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Ovarian steroidogenesis | hsa04913 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Progesterone-mediated oocyte maturation | hsa04914 | Affiliated Target |
|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Prolactin signaling pathway | hsa04917 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Regulation of lipolysis in adipocytes | hsa04923 | Affiliated Target |
|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Aldosterone-regulated sodium reabsorption | hsa04960 | Affiliated Target |
|
| Class: Organismal Systems => Excretory system | Pathway Hierarchy | ||
| Click to Show/Hide the Information of Affiliated Human Pathways | |||
| Degree | 44 | Degree centrality | 4.73E-03 | Betweenness centrality | 9.78E-03 |
|---|---|---|---|---|---|
| Closeness centrality | 2.56E-01 | Radiality | 1.44E+01 | Clustering coefficient | 1.25E-01 |
| Neighborhood connectivity | 3.50E+01 | Topological coefficient | 4.61E-02 | Eccentricity | 11 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating Transcription Factors | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | 2005 approvals: Safety first. Nature Reviews Drug Discovery 5, 92-93 (February 2006). | |||||
| REF 2 | Radium 223 dichloride for prostate cancer treatment. Drug Des Devel Ther. 2017 Sep 6;11:2643-2651. | |||||
| REF 3 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||||
| REF 4 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. | |||||
| REF 5 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7572). | |||||
| REF 6 | ClinicalTrials.gov (NCT00411892) Effect of Inhaled Insulin (AERx iDMS) on Blood Glucose Control in Type 2 Diabetes. U.S. National Institutes of Health. | |||||
| REF 7 | Clinical pipeline report, company report or official report of Sanofi. | |||||
| REF 8 | ClinicalTrials.gov (NCT01792284) A Study of LY2605541 in Participants With Type 1 Diabetes Mellitus. U.S. National Institutes of Health. | |||||
| REF 9 | ClinicalTrials.gov (NCT02302716) A Study of LY2963016 Compared to LANTUS in Adult Participants With Type 2 Diabetes Mellitus. U.S. National Institutes of Health. | |||||
| REF 10 | ClinicalTrials.gov (NCT02273180) Comparison of SAR342434 to Humalog as the Rapid Acting Insulin in Adult Patients With Type 1 Diabetes Mellitus Also Using Insulin Glargine. U.S. National Institutes of Health. | |||||
| REF 11 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 12 | ClinicalTrials.gov (NCT02470039) Trial to Compare NNC0123-0000-0338 in a Tablet Formulation and Insulin Glargine in Subjects With Type 2 Diabetes Currently Treated With Oral Antidiabetic Therapy. | |||||
| REF 13 | ClinicalTrials.gov (NCT01053728) Study of How Single Rising Doses of SAR161271 Are Absorbed and Act in Patients With Type 1 Diabetes Mellitus (T1DM). U.S. National Institutes of Health. | |||||
| REF 14 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800033163) | |||||
| REF 15 | ClinicalTrials.gov (NCT00057499) Evaluation of a Diabetes Vaccine in Newly Diagnosed Diabetics. U.S. National Institutes of Health. | |||||
| REF 16 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800024901) | |||||
| REF 17 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800037853) | |||||
| REF 18 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800036594) | |||||
| REF 19 | Proinsulin peptide immunotherapy in type 1 diabetes: report of a first-in-man Phase I safety study. Clin Exp Immunol. 2009 Feb;155(2):156-65. | |||||
| REF 20 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800011773) | |||||
| REF 21 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800004396) | |||||
| REF 22 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800013224) | |||||
| REF 23 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800015749) | |||||
| REF 24 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||||
| REF 25 | LY2605541--a preferential hepato-specific insulin analogue. Diabetes. 2014 Feb;63(2):390-2. | |||||
| REF 26 | Efficacy and safety of LY2963016 insulin glargine compared with insulin glargine (Lantus ) in patients with type 1 diabetes in a randomized controlled trial: the ELEMENT 1 study. Diabetes Obes Metab.2015 Aug;17(8):726-33. | |||||
| REF 27 | Biosimilar insulins: a European perspective. Diabetes Obes Metab. 2015 May; 17(5): 445-451. | |||||
| REF 28 | Clinical pipeline report, company report or official report of Mercia Pharma Inc. | |||||
| REF 29 | ClinicalTrials.gov (NCT01334034) Safety of NNC 0123-0000-0338 in Healthy Subjects. U.S. National Institutes of Health. | |||||
| REF 30 | Clinical pipeline report, company report or official report of sanofi-aventis. | |||||
| REF 31 | The future of basal insulin supplementation. Diabetes Technol Ther. 2011 Jun;13 Suppl 1:S103-8. | |||||
| REF 32 | Prevention of Type 1 Diabetes Mellitus using a Novel Vaccine. Ther Adv Endocrinol Metab. 2011 February; 2(1): 9-16. | |||||
| REF 33 | Napo Receives Commitments for a Private Placement of Common Shares and Enters into Binding Letter of Intent for Intellectual Property License. Napo. JANUARY 08, 2007 | |||||
| REF 34 | Company report (Novonordisk) | |||||
| REF 35 | Novo Nordisk increased operating profit in local currencies by 15% in the first quarter of 2014 | |||||
| REF 36 | Synergistic antihyperglycemic effects between plant-derived oleanolic acid and insulin in streptozotocin-induced diabetic rats. Ren Fail. 2010;32(7):832-9. | |||||
| REF 37 | No effect of the altered peptide ligand NBI-6024 on beta-cell residual function and insulin needs in new-onset type 1 diabetes.Diabetes Care.2009 Nov;32(11):2036-40. | |||||
| REF 38 | Immunological characterization and therapeutic activity of an altered-peptide ligand, NBI-6024, based on the immunodominant type 1 diabetes autoantigen insulin B-chain (9-23) peptide. Diabetes. 2002 Jul;51(7):2126-34. | |||||
| REF 39 | Beneficial insulin-sensitizing and vascular effects of S15261 in the insulin-resistant JCR:LA-cp rat. J Pharmacol Exp Ther. 2000 Nov;295(2):753-60. | |||||
| REF 40 | Degludec insulin: A novel basal insulin. Indian J Endocrinol Metab. 2011 July; 15(Suppl1): S12-S16. | |||||
| REF 41 | ClinicalTrials.gov (NCT01120912) Safety and Efficacy of Single Administration of Oshadi Oral Insulin in Type I Diabetes Patients. U.S. National Institutes of Health. | |||||
| REF 42 | Long-term comparison of human insulin analogue B10Asp and soluble human insulin in IDDM patients on a basal/bolus insulin regimen. Diabetologia. 1995 May;38(5):592-8. | |||||
| REF 43 | Structural characterization of insulin NPH formulations. Eur J Pharm Sci. 2007 Apr;30(5):414-23. | |||||
| REF 44 | Computational and structural evidence for neurotransmitter-mediated modulation of the oligomeric states of human insulin in storage granules. J Biol Chem. 2017 May 19;292(20):8342-8355. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

