Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0FV3V
|
|||
| Former ID |
DAP001115
|
|||
| Drug Name |
Mepenzolate
|
|||
| Synonyms |
Cantil; Cantilaque; Cantilon; Cantril; Colibantil; Colopiril; Colum; Delevil; Eftoron; Gastropidil; Glycophenylate; Mepenzolon; Tralanta; Trancolon; Trokonil; Bromure de mepenzolate; Bromuro de mepenzolato; MEPENZOLATE BROMIDE; Mepenzolate Methylbromide; Mepenzolati bromidum; Mepenzolic acid; Mepenzolone Bromide; JB 340; Bromure de mepenzolate [INN-French]; Bromuro de mepenzolato [INN-Spanish]; Cantil (TN); Mepenzolati bromidum [INN-Latin]; Mepenzolic acid, bromine salt; Mepenzolate bromide (JP15/INN); Mepenzolate bromide [INN:BAN:JAN]; N-Methyl-3-piperidyl benzilate methobromide; N-Methyl-3-piperidyl benzilate methyl bromide; N-Methyl-3-piperidyldiphenylglycolate methobromide; N-methyl-3-piperidylbenzilate methyl bromide; Benzilic acid, 1-methyl-3-piperidyl ester methobromide; Benzilic acid, ester with 3-hydroxy-1,1-dimethylpiperidinium bromide; Piperidinium, 3-[(hydroxydiphenylacetyl)oxy]-1,1-dimethyl-, bromide; Piperidinium, 3-hydroxy-1,1-dimethyl-, benzilate (ester); Piperidinium, 3-hydroxy-1,1-dimethyl-, bromide, benzilate; Piperidinium, 3-((hydroxydiphenylacetyl)oxy)-1,1-dimethyl-(VAN); Piperidinium, 3-((hydroxydiphenylacetyl)oxy)-1,1-dimethyl-, bromide; (1,1-dimethylpiperidin-1-ium-3-yl) 2-hydroxy-2,2-diphenylacetate; (1,1-dimethylpiperidin-1-ium-3-yl) 2-hydroxy-2,2-diphenylacetate bromide; 1-Methyl-3-piperidyl benzilate methyl bromide; 1-Methyl-3-piperidyl benzilate methylbromide; 2-(1,1-dimethylpiperidin-1-ium-3-yl)oxy-2-oxo-1,1-diphenylethanolate; 2-[(1,1-dimethylpiperidinium-3-yl)oxy]-2-oxo-1,1-diphenylethanolate; 3-((Hydroxydiphenylacetyl)oxy)-1,1-dimethylpiperidinium bromide; 3-Hydroxy-1,1-dimethylpiperidinium bromide benzilate; 3-{[hydroxy(diphenyl)acetyl]oxy}-1,1-dimethylpiperidinium bromide
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Gastrointestinal disease [ICD-11: DE2Z] | Approved | [1], [2] | |
| Peptic ulcer [ICD-11: DA61] | Approved | [1], [2] | ||
| Therapeutic Class |
Parasympatholytics
|
|||
| Structure |
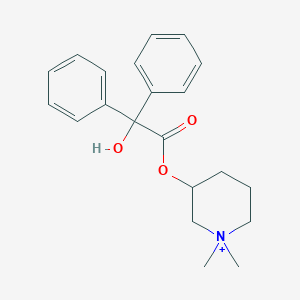 |
Download2D MOL |
||
| Formula |
C21H26NO3+
|
|||
| Canonical SMILES |
C[N+]1(CCCC(C1)OC(=O)C(C2=CC=CC=C2)(C3=CC=CC=C3)O)C
|
|||
| InChI |
1S/C21H26NO3/c1-22(2)15-9-14-19(16-22)25-20(23)21(24,17-10-5-3-6-11-17)18-12-7-4-8-13-18/h3-8,10-13,19,24H,9,14-16H2,1-2H3/q+1
|
|||
| InChIKey |
GKNPSSNBBWDAGH-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 25990-43-6
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
538809, 7847785, 8149412, 8154122, 10321201, 14758204, 26611806, 26680676, 26748120, 26748121, 29225438, 48416211, 49648464, 50017627, 57323461, 80305131, 85273696, 91011337, 92124149, 92125651, 92307228, 92714640, 99301724, 103680225, 103913681, 104134258, 104170016, 104312126, 117370002, 121362619, 121363322, 124637950, 124799975, 126629471, 126666034, 134971984, 144075111, 144203918, 152101554, 162224989, 163414851, 164761771, 170465214, 172080641, 172882153, 178125529, 179148948, 184580881, 223680849, 226474501
|
|||
| ChEBI ID |
CHEBI:94411
|
|||
| ADReCS Drug ID | BADD_D01382 ; BADD_D01383 | |||
| SuperDrug ATC ID |
A03AB12
|
|||
| SuperDrug CAS ID |
cas=025990436
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Muscarinic acetylcholine receptor M5 (CHRM5) | Target Info | Antagonist | [3] |
| KEGG Pathway | Calcium signaling pathway | |||
| Neuroactive ligand-receptor interaction | ||||
| Cholinergic synapse | ||||
| Regulation of actin cytoskeleton | ||||
| Panther Pathway | Alzheimer disease-amyloid secretase pathway | |||
| Heterotrimeric G-protein signaling pathway-Gq alpha and Go alpha mediated pathway | ||||
| Reactome | Muscarinic acetylcholine receptors | |||
| G alpha (q) signalling events | ||||
| WikiPathways | Monoamine GPCRs | |||
| Calcium Regulation in the Cardiac Cell | ||||
| Regulation of Actin Cytoskeleton | ||||
| GPCRs, Class A Rhodopsin-like | ||||
| Gastrin-CREB signalling pathway via PKC and MAPK | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 010679. | |||
| REF 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 3 | Isolation of cholinergic active ingredients in aqueous extracts of Mareya micrantha using the longitudinal muscle of isolated guinea-pig ileum as a pharmacological activity marker. J Ethnopharmacol. 1995 Mar;45(3):215-22. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

