Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T16156
(Former ID: TTDS00524)
|
|||||
| Target Name |
Thrombopoietin receptor (MPL)
|
|||||
| Synonyms |
TPOR; TPO-R; Proto-oncogene c-Mpl; Myeloproliferative leukemia protein; CD110 antigen; CD110; C-mpl
Click to Show/Hide
|
|||||
| Gene Name |
MPL
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 1 Target-related Diseases | + | ||||
| 1 | Thrombocytopenia [ICD-11: 3B64] | |||||
| Function |
May represent a regulatory molecule specific for TPO-R-dependent immune responses. Receptor for thrombopoietin that acts as a primary regulator of megakaryopoiesis and platelet production.
Click to Show/Hide
|
|||||
| BioChemical Class |
Cytokine receptor
|
|||||
| UniProt ID | ||||||
| Sequence |
MPSWALFMVTSCLLLAPQNLAQVSSQDVSLLASDSEPLKCFSRTFEDLTCFWDEEEAAPS
GTYQLLYAYPREKPRACPLSSQSMPHFGTRYVCQFPDQEEVRLFFPLHLWVKNVFLNQTR TQRVLFVDSVGLPAPPSIIKAMGGSQPGELQISWEEPAPEISDFLRYELRYGPRDPKNST GPTVIQLIATETCCPALQRPHSASALDQSPCAQPTMPWQDGPKQTSPSREASALTAEGGS CLISGLQPGNSYWLQLRSEPDGISLGGSWGSWSLPVTVDLPGDAVALGLQCFTLDLKNVT CQWQQQDHASSQGFFYHSRARCCPRDRYPIWENCEEEEKTNPGLQTPQFSRCHFKSRNDS IIHILVEVTTAPGTVHSYLGSPFWIHQAVRLPTPNLHWREISSGHLELEWQHPSSWAAQE TCYQLRYTGEGHQDWKVLEPPLGARGGTLELRPRSRYRLQLRARLNGPTYQGPWSSWSDP TRVETATETAWISLVTALHLVLGLSAVLGLLLLRWQFPAHYRRLRHALWPSLPDLHRVLG QYLRDTAALSPPKATVSDTCEEVEPSLLEILPKSSERTPLPLCSSQAQMDYRRLQPSCLG TMPLSVCPPMAESGSCCTTHIANHSYLPLSYWQQP Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| HIT2.0 ID | T88D60 | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 4 Approved Drugs | + | ||||
| 1 | Eltrombopag | Drug Info | Approved | Thrombocytopenia | [3], [4] | |
| 2 | Lusutrombopag | Drug Info | Approved | Thrombocytopenia | [2] | |
| 3 | Revolade/Promacta | Drug Info | Approved | Idiopathic thrombocytopenic purpura | [5] | |
| 4 | Romiplostim | Drug Info | Approved | Thrombocytopenia | [4], [6] | |
| Clinical Trial Drug(s) | [+] 3 Clinical Trial Drugs | + | ||||
| 1 | LGD-4665 | Drug Info | Phase 2 | Immune thrombocytopenic purpura | [1] | |
| 2 | BVI-007 | Drug Info | Phase 1 | Thrombotic stroke | [7] | |
| 3 | NIP-004 | Drug Info | Phase 1 | Thrombocytopenia | [8] | |
| Discontinued Drug(s) | [+] 3 Discontinued Drugs | + | ||||
| 1 | RhTPO | Drug Info | Discontinued in Phase 3 | Idiopathic thrombocytopenic purpura | [1] | |
| 2 | Totrombopag | Drug Info | Discontinued in Phase 1 | Thrombocytopenia | [1] | |
| 3 | Promegapoietin | Drug Info | Terminated | Bone marrow transplantation | [9] | |
| Mode of Action | [+] 3 Modes of Action | + | ||||
| Agonist | [+] 9 Agonist drugs | + | ||||
| 1 | Eltrombopag | Drug Info | [1] | |||
| 2 | Lusutrombopag | Drug Info | [2] | |||
| 3 | Revolade/Promacta | Drug Info | [10] | |||
| 4 | LGD-4665 | Drug Info | [1] | |||
| 5 | NIP-004 | Drug Info | [12] | |||
| 6 | RhTPO | Drug Info | [1] | |||
| 7 | Totrombopag | Drug Info | [10] | |||
| 8 | SB-559457 | Drug Info | [11] | |||
| 9 | STS-T4 | Drug Info | [11] | |||
| Modulator | [+] 6 Modulator drugs | + | ||||
| 1 | Romiplostim | Drug Info | [4] | |||
| 2 | Promegapoietin | Drug Info | [13] | |||
| 3 | Non-peptide TPO mimics | Drug Info | [11] | |||
| 4 | RI-TPO | Drug Info | [14] | |||
| 5 | SE-ET-TP020d | Drug Info | [14] | |||
| 6 | ThromboMer | Drug Info | [11] | |||
| Antagonist | [+] 2 Antagonist drugs | + | ||||
| 1 | BVI-007 | Drug Info | [11] | |||
| 2 | HL-034 | Drug Info | [11] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Pathway Affiliation
Biological Network Descriptors
|
|
| Protein Name | Pfam ID | Percentage of Identity (%) | E value |
|---|---|---|---|
| Interleukin 21 receptor (IL21R) | 29.412 (45/153) | 4.67E-04 | |
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Cytokine-cytokine receptor interaction | hsa04060 | Affiliated Target |
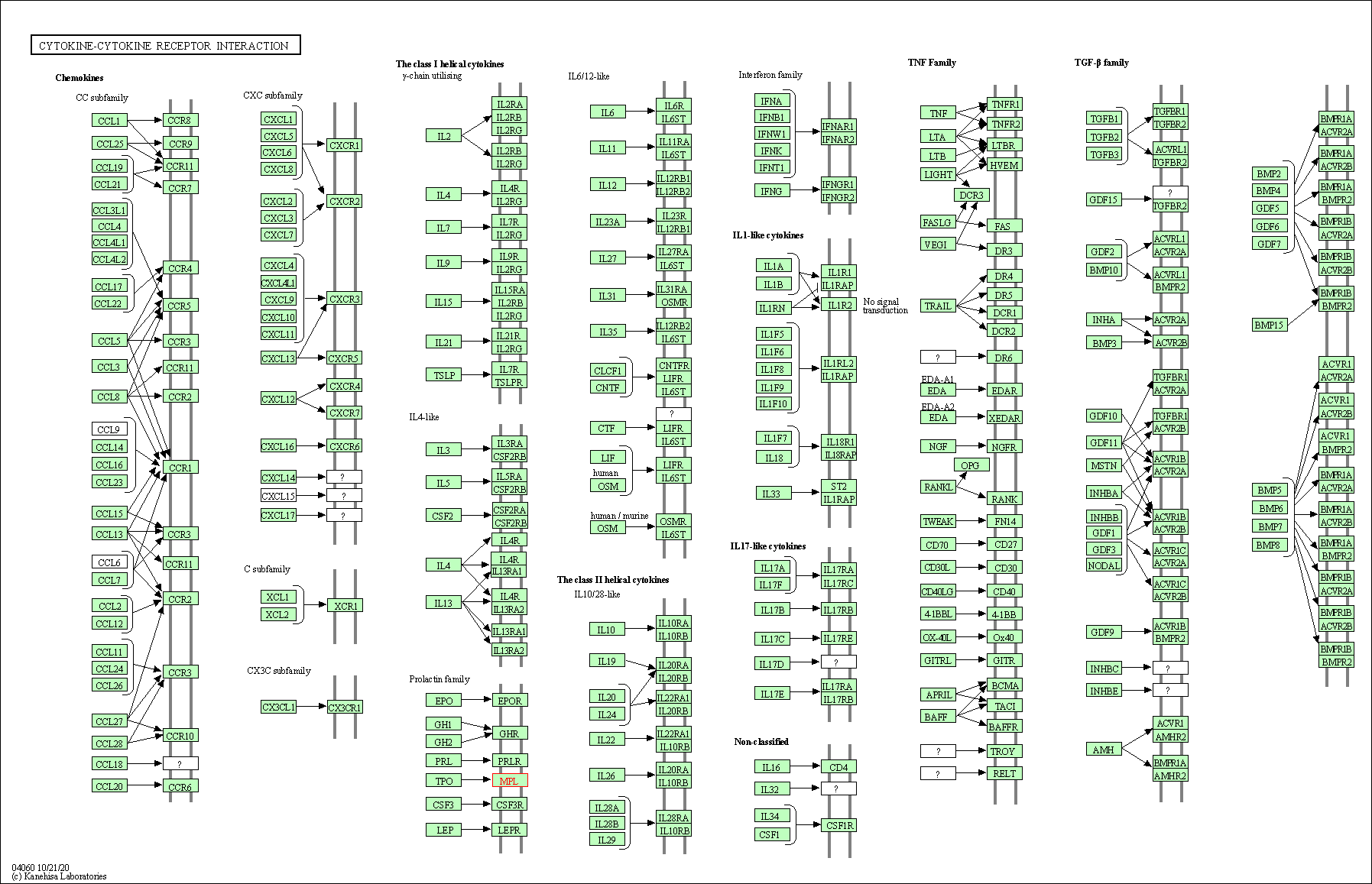
|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
| JAK-STAT signaling pathway | hsa04630 | Affiliated Target |
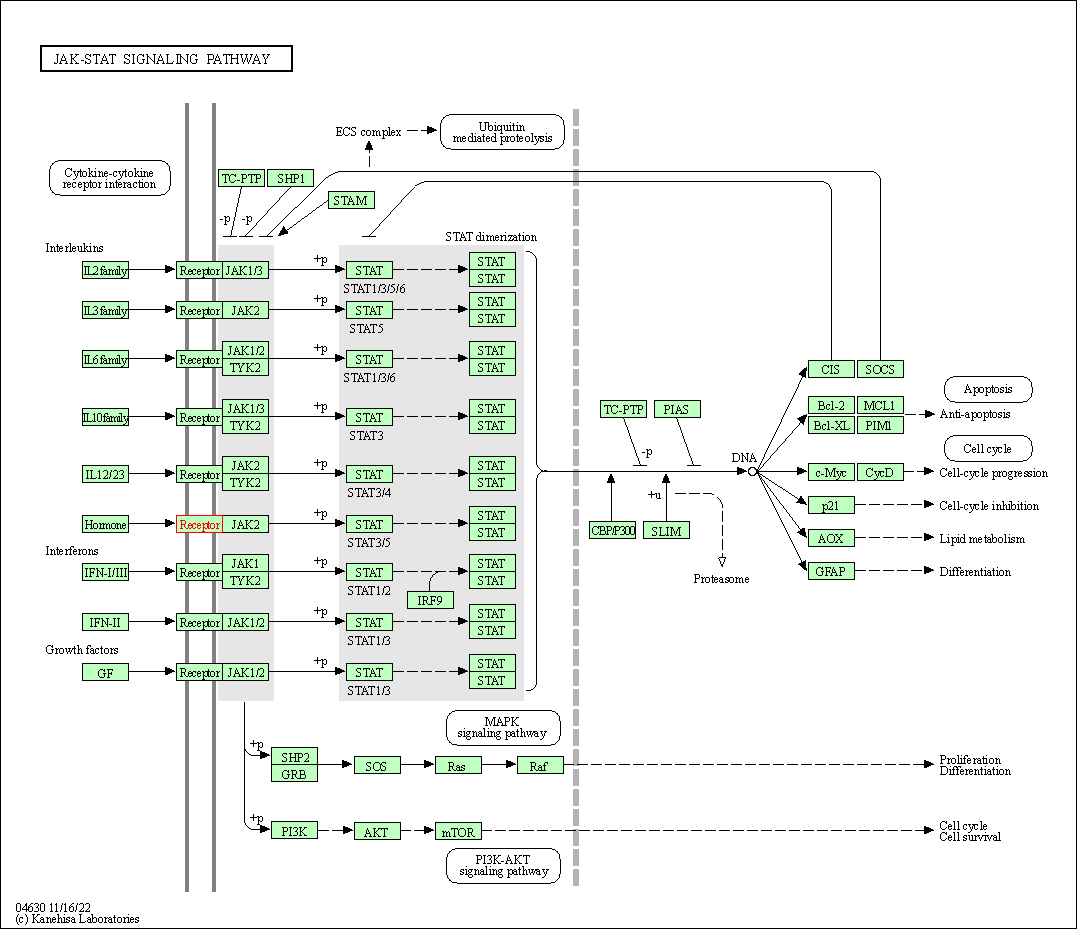
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Degree | 7 | Degree centrality | 7.52E-04 | Betweenness centrality | 4.31E-05 |
|---|---|---|---|---|---|
| Closeness centrality | 2.27E-01 | Radiality | 1.40E+01 | Clustering coefficient | 3.81E-01 |
| Neighborhood connectivity | 6.07E+01 | Topological coefficient | 2.37E-01 | Eccentricity | 12 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) | ||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 2 KEGG Pathways | + | ||||
| 1 | Cytokine-cytokine receptor interaction | |||||
| 2 | Jak-STAT signaling pathway | |||||
| WikiPathways | [+] 1 WikiPathways | + | ||||
| 1 | Platelet Aggregation (Plug Formation) | |||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Emerging drugs for idiopathic thrombocytopenic purpura in adults. Expert Opin Emerg Drugs. 2008 Jun;13(2):237-54. | |||||
| REF 2 | 2018 FDA drug approvals.Nat Rev Drug Discov. 2019 Feb;18(2):85-89. | |||||
| REF 3 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6961). | |||||
| REF 4 | 2008 FDA drug approvals. Nat Rev Drug Discov. 2009 Feb;8(2):93-6. | |||||
| REF 5 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 022291. | |||||
| REF 6 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6974). | |||||
| REF 7 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800027040) | |||||
| REF 8 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800027636) | |||||
| REF 9 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800010450) | |||||
| REF 10 | Clinical pipeline report, company report or official report of GlaxoSmithKline (2009). | |||||
| REF 11 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 1722). | |||||
| REF 12 | A novel nonpeptidyl human c-Mpl activator stimulates human megakaryopoiesis and thrombopoiesis. Blood. 2006 Jun 1;107(11):4300-7. | |||||
| REF 13 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||||
| REF 14 | The ChEMBL database in 2017. Nucleic Acids Res. 2017 Jan 4;45(D1):D945-D954. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

