Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T19784
(Former ID: TTDR01048)
|
|||||
| Target Name |
Somatotropin (GH1)
|
|||||
| Synonyms |
Pituitary growth hormone; Growth hormone 1; Growth hormone; GH-N; GH
Click to Show/Hide
|
|||||
| Gene Name |
GH1
|
|||||
| Target Type |
Clinical trial target
|
[1] | ||||
| Disease | [+] 2 Target-related Diseases | + | ||||
| 1 | Multiple structural anomalies syndrome [ICD-11: LD2F] | |||||
| 2 | Pituitary gland disorder [ICD-11: 5A60-5A61] | |||||
| Function |
Plays an important role in growth control. Its major role in stimulating body growth is to stimulate the liver and other tissues to secrete IGF-1. It stimulates both the differentiation and proliferation of myoblasts. It also stimulates amino acid uptake and protein synthesis in muscle and other tissues.
Click to Show/Hide
|
|||||
| BioChemical Class |
Somatotropin/prolactin
|
|||||
| UniProt ID | ||||||
| Sequence |
MATGSRTSLLLAFGLLCLPWLQEGSAFPTIPLSRLFDNAMLRAHRLHQLAFDTYQEFEEA
YIPKEQKYSFLQNPQTSLCFSESIPTPSNREETQQKSNLELLRISLLLIQSWLEPVQFLR SVFANSLVYGASDSNVYDLLKDLEEGIQTLMGRLEDGSPRTGQIFKQTYSKFDTNSHNDD ALLKNYGLLYCFRKDMDKVETFLRIVQCRSVEGSCGF Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | PDB | ||||
| HIT2.0 ID | T24VFB | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Clinical Trial Drug(s) | [+] 3 Clinical Trial Drugs | + | ||||
| 1 | HGH-CTP | Drug Info | Phase 3 | Growth hormone deficiency | [4] | |
| 2 | Somatropin intranasal rhGH | Drug Info | Phase 2 | Growth hormone deficiency | [5] | |
| 3 | YPEG-Somatropin | Drug Info | Phase 1 | Growth hormone deficiency | [6] | |
| Discontinued Drug(s) | [+] 1 Discontinued Drugs | + | ||||
| 1 | ARX-201 | Drug Info | Discontinued in Phase 2 | Growth failure | [7] | |
| Mode of Action | [+] 2 Modes of Action | + | ||||
| Agonist | [+] 1 Agonist drugs | + | ||||
| 1 | HGH-CTP | Drug Info | [1] | |||
| Modulator | [+] 3 Modulator drugs | + | ||||
| 1 | Somatropin intranasal rhGH | Drug Info | [8] | |||
| 2 | YPEG-Somatropin | Drug Info | [9] | |||
| 3 | ARX-201 | Drug Info | [10] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: (2S)-6-acetamido-2-[[2-[2-[2-[[(4R)-4-carboxy-4-[[(4S)-4-carboxy-4-[[2-[2-[2-[4-[16-(2H-tetrazol-5-yl)hexadecanoylsulfamoyl]butanoylamino]ethoxy]ethoxy]acetyl]amino]butanoyl]amino]butanoyl]amino]ethoxy]ethoxy]acetyl]amino]hexanoic acid | Ligand Info | |||||
| Structure Description | Ternary complex of FcRn ectodomain, FcRn binding optimised human serum albumin and the human growth hormone derivative somapacitan | PDB:6QIO | ||||
| Method | X-ray diffraction | Resolution | 1.95 Å | Mutation | Yes | [11] |
| PDB Sequence |
C
|
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Cytokine-cytokine receptor interaction | hsa04060 | Affiliated Target |
|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
| Neuroactive ligand-receptor interaction | hsa04080 | Affiliated Target |

|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
| PI3K-Akt signaling pathway | hsa04151 | Affiliated Target |
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| JAK-STAT signaling pathway | hsa04630 | Affiliated Target |
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Growth hormone synthesis, secretion and action | hsa04935 | Affiliated Target |
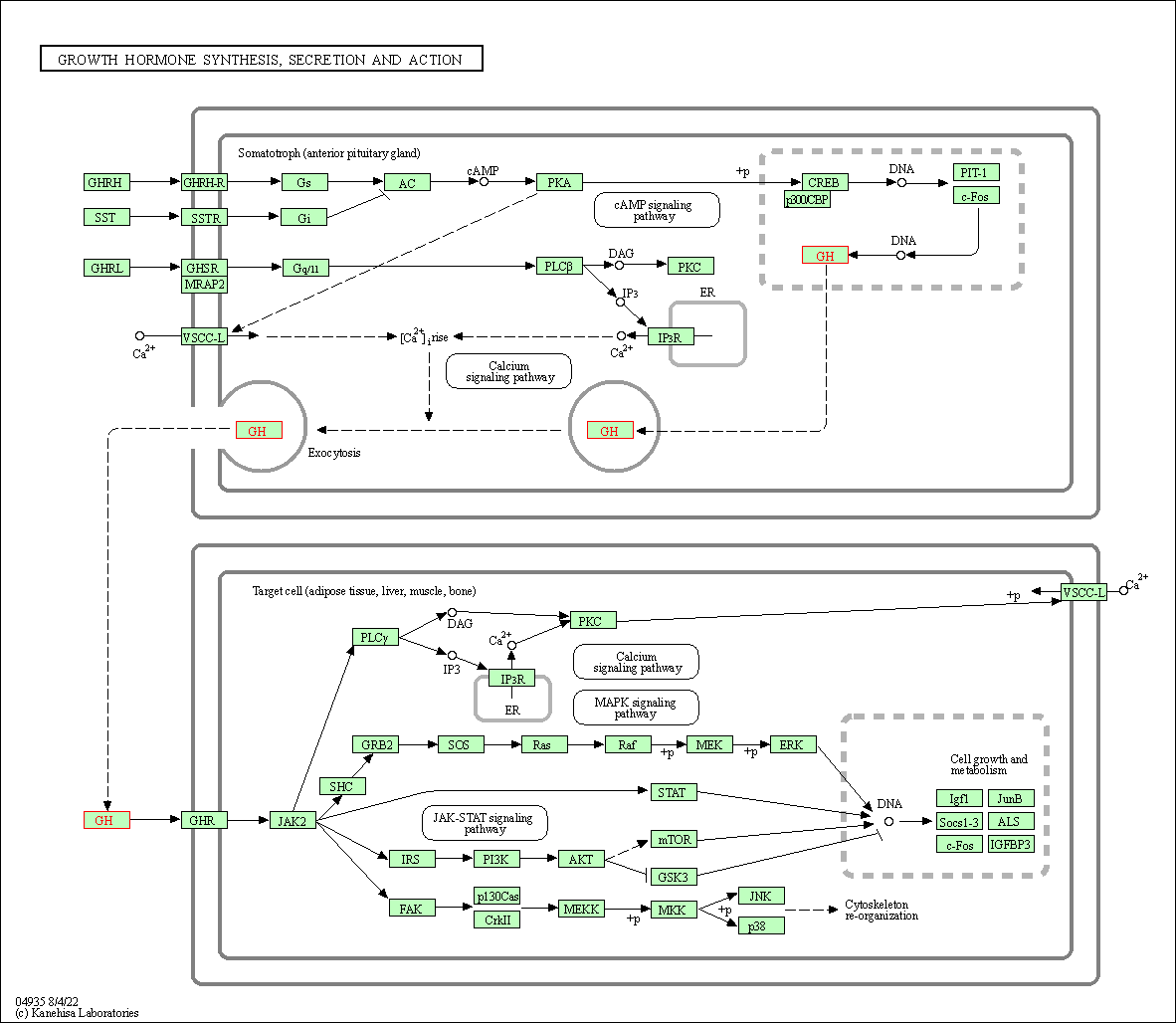
|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Degree | 3 | Degree centrality | 3.22E-04 | Betweenness centrality | 3.76E-06 |
|---|---|---|---|---|---|
| Closeness centrality | 1.78E-01 | Radiality | 1.29E+01 | Clustering coefficient | 0.00E+00 |
| Neighborhood connectivity | 8.33E+00 | Topological coefficient | 3.86E-01 | Eccentricity | 13 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating Transcription Factors | ||||||
| Target-interacting Proteins | ||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 4 KEGG Pathways | + | ||||
| 1 | Cytokine-cytokine receptor interaction | |||||
| 2 | Neuroactive ligand-receptor interaction | |||||
| 3 | PI3K-Akt signaling pathway | |||||
| 4 | Jak-STAT signaling pathway | |||||
| Reactome | [+] 2 Reactome Pathways | + | ||||
| 1 | Prolactin receptor signaling | |||||
| 2 | Growth hormone receptor signaling | |||||
| WikiPathways | [+] 5 WikiPathways | + | ||||
| 1 | Endochondral Ossification | |||||
| 2 | Prolactin receptor signaling | |||||
| 3 | Growth hormone receptor signaling | |||||
| 4 | Adipogenesis | |||||
| 5 | Synthesis, Secretion, and Deacylation of Ghrelin | |||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Developments in human growth hormone preparations: sustained-release, prolonged half-life, novel injection devices, and alternative delivery routes. Int J Nanomedicine. 2014; 9: 3527-3538. | |||||
| REF 2 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800016005) | |||||
| REF 3 | ClinicalTrials.gov (NCT02339090) Comparison of Long-acting Growth Hormone VRS-317 to Daily Growth Hormone in Pediatric GHD Patients. U.S. National Institutes of Health. | |||||
| REF 4 | ClinicalTrials.gov (NCT01909479) A Phase 3, Multicenter Study Designed To Evaluate The Efficacy And Safety Of A Long Acting Hgh Product (Mod-4023) In Adult Subjects With Growth Hormone Deficiency. U.S. National Institutes of Health. | |||||
| REF 5 | ClinicalTrials.gov (NCT00837863) Study of Weekly ALTU-238 Compared With Daily Nutropin AQ in Prepubertal Children With Growth Hormone Deficiency. U.S. National Institutes of Health. | |||||
| REF 6 | ClinicalTrials.gov (NCT01339182) Safety, Tolerability, Pharmacokinetics and Pharmacodynamics Study of Pegylated-Somatropin in Healthy Volunteers. U.S. National Institutes of Health. | |||||
| REF 7 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800022324) | |||||
| REF 8 | Long-term efficacy and safety of somatropin for adult growth hormone deficiency.Treat Endocrinol.2003;2(2):109-20. | |||||
| REF 9 | Somatropin therapy in adults with Prader-Willi syndrome. Treat Endocrinol. 2004;3(3):153-60. | |||||
| REF 10 | Company report (Avarx) | |||||
| REF 11 | Identification of Binding Sites on Human Serum Albumin for Somapacitan, a Long-Acting Growth Hormone Derivative. Biochemistry. 2020 Apr 14;59(14):1410-1419. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

