Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T28887
(Former ID: TTDR01337)
|
|||||
| Target Name |
Histone deacetylase 8 (HDAC8)
|
|||||
| Synonyms |
Histone deacetylase-8; HDACL1; HD8; CDA07
Click to Show/Hide
|
|||||
| Gene Name |
HDAC8
|
|||||
| Target Type |
Clinical trial target
|
[1] | ||||
| Disease | [+] 1 Target-related Diseases | + | ||||
| 1 | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||||
| Function |
Gives a tag for epigenetic repression and plays an important role in transcriptional regulation, cell cycle progression and developmental events. Histone deacetylases act via the formation of large multiprotein complexes. Also involved in the deacetylation of cohesin complex protein SMC3 regulating release of cohesin complexes from chromatin. May play a role in smooth muscle cell contractility. Responsible for the deacetylation of lysine residues on the N-terminal part of the core histones (H2A, H2B, H3 and H4).
Click to Show/Hide
|
|||||
| BioChemical Class |
Carbon-nitrogen hydrolase
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 3.5.1.98
|
|||||
| Sequence |
MEEPEEPADSGQSLVPVYIYSPEYVSMCDSLAKIPKRASMVHSLIEAYALHKQMRIVKPK
VASMEEMATFHTDAYLQHLQKVSQEGDDDHPDSIEYGLGYDCPATEGIFDYAAAIGGATI TAAQCLIDGMCKVAINWSGGWHHAKKDEASGFCYLNDAVLGILRLRRKFERILYVDLDLH HGDGVEDAFSFTSKVMTVSLHKFSPGFFPGTGDVSDVGLGKGRYYSVNVPIQDGIQDEKY YQICESVLKEVYQAFNPKAVVLQLGADTIAGDPMCSFNMTPVGIGKCLKYILQWQLATLI LGGGGYNLANTARCWTYLTGVILGKTLSSEIPDHEFFTAYGPDYVLEITPSCRPDRNEPH RIQQILNYIKGNLKHVV Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | PDB | ||||
| HIT2.0 ID | T90RVD | |||||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Vorinostat | Ligand Info | |||||
| Structure Description | Crystal structure of human histone deacetylase 8 (HDAC8) I45T mutation complexed with SAHA | PDB:7JVU | ||||
| Method | X-ray diffraction | Resolution | 1.50 Å | Mutation | Yes | [20] |
| PDB Sequence |
LVPVYIYSPE
23 YVSMCDSLAK33 IPKRASMVHS43 LTEAYALHKQ53 MRIVKPKVAS63 MEEMATFHTD 73 AYLQHLQKVS83 QEGDDDHPDS93 IEYGLGYDCP103 ATEGIFDYAA113 AIGGATITAA 123 QCLIDGMCKV133 AINWSGGWHH143 AKKDEASGFC153 YLNDAVLGIL163 RLRRKFERIL 173 YVDLDLHHGD183 GVEDAFSFTS193 KVMTVSLHKF203 SPGFFPGTGD213 VSDVGLGKGR 223 YYSVNVPIQD233 GIQDEKYYQI243 CESVLKEVYQ253 AFNPKAVVLQ263 LGADTIAGDP 273 MCSFNMTPVG283 IGKCLKYILQ293 WQLATLILGG303 GGYNLANTAR313 CWTYLTGVIL 323 GKTLSSEIPD333 HEFFTAYGPD343 YVLEITPSCR353 PDRNEPHRIQ363 QILNYIKGNL 373 KHVVI
|
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target and Ligand Pair | ||||||
| Ligand Name: JNJ-26481585 | Ligand Info | |||||
| Structure Description | Crystal structure of a human HDAC8 L6 loop mutant complexed with Quisinostat | PDB:6HSK | ||||
| Method | X-ray diffraction | Resolution | 2.10 Å | Mutation | No | [21] |
| PDB Sequence |
LVPVYIYSPE
23 YVSMCDSLAK33 IPKRASMVHS43 LIEAYALHKQ53 MRIVKPKVAS63 MEEMATFHTD 73 AYLQHLQKVS83 QEGDDDHPDS93 IEYGLGYDCP103 ATEGIFDYAA113 AIGGATITAA 123 QCLIDGMCKV133 AINWSGGWHH143 AKKDEASGFC153 YLNDAVLGIL163 RLRRKFERIL 173 YVDLDLHHGD183 GVEDAFSFTS193 KVMTVSLHKF203 SPGFFPGTGD213 VSDVGLGKGR 223 YYSVNVPIQD233 GIQDEKYYQI243 CESVLKEVYQ253 AFNPKAVVLQ263 LGADTISGDR 273 LGCFNMTPVG283 IGKCLKYILQ293 WQLATLILGG303 GGYNLANTAR313 CWTYLTGVIL 323 GKTLSSEIPD333 HEFFTAYGPD343 YVLEITPSCR353 PDRNEPHRIQ363 QILNYIKGNL 373 KHVV
|
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Neutrophil extracellular trap formation | hsa04613 | Affiliated Target |
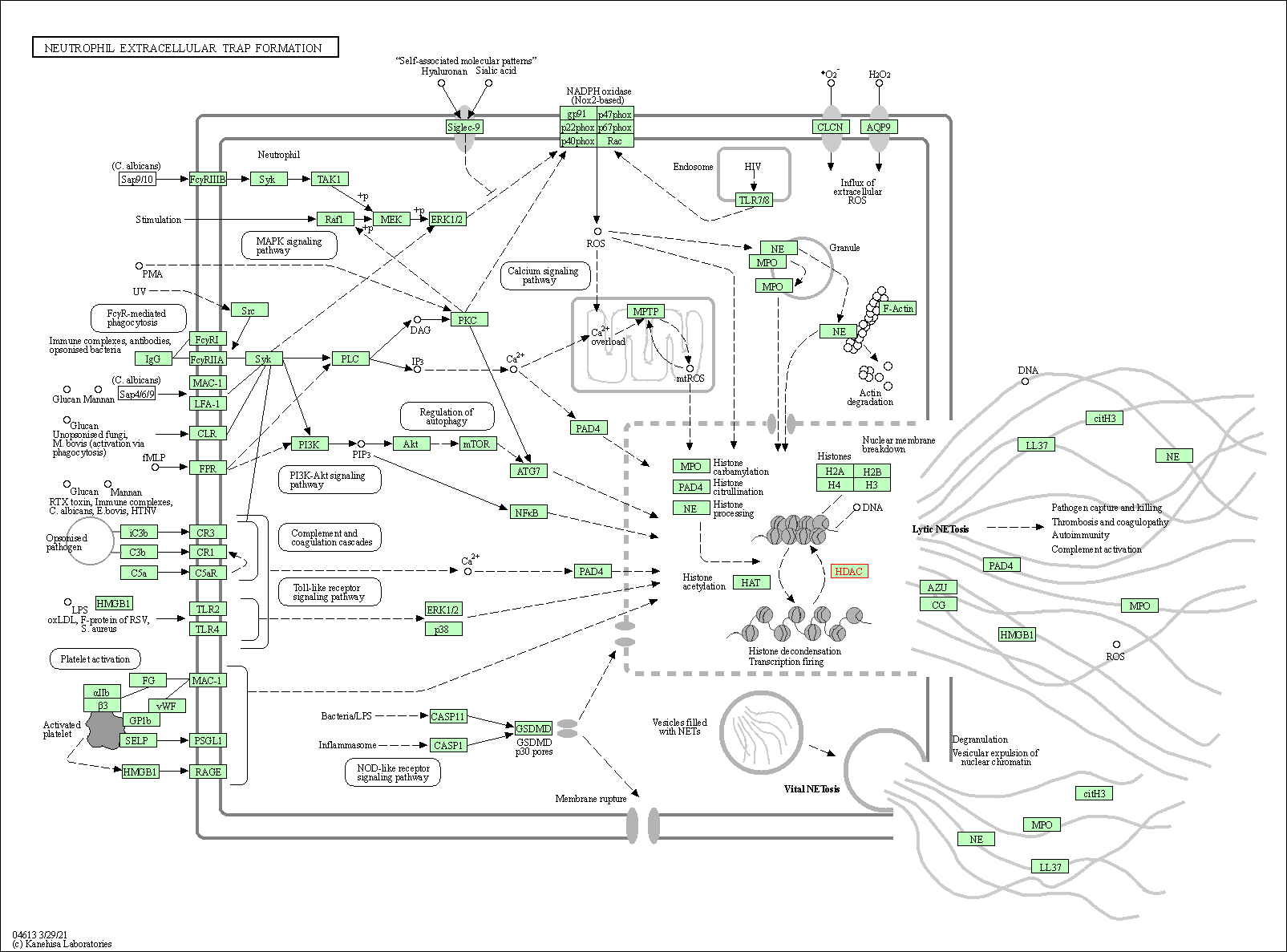
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Degree | 8 | Degree centrality | 8.59E-04 | Betweenness centrality | 6.03E-05 |
|---|---|---|---|---|---|
| Closeness centrality | 2.01E-01 | Radiality | 1.35E+01 | Clustering coefficient | 3.93E-01 |
| Neighborhood connectivity | 2.58E+01 | Topological coefficient | 2.39E-01 | Eccentricity | 12 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 2 KEGG Pathways | + | ||||
| 1 | Alcoholism | |||||
| 2 | Viral carcinogenesis | |||||
| Panther Pathway | [+] 1 Panther Pathways | + | ||||
| 1 | Wnt signaling pathway | |||||
| PID Pathway | [+] 1 PID Pathways | + | ||||
| 1 | Signaling events mediated by HDAC Class I | |||||
| Reactome | [+] 4 Reactome Pathways | + | ||||
| 1 | NOTCH1 Intracellular Domain Regulates Transcription | |||||
| 2 | Constitutive Signaling by NOTCH1 PEST Domain Mutants | |||||
| 3 | Constitutive Signaling by NOTCH1 HD+PEST Domain Mutants | |||||
| 4 | HDACs deacetylate histones | |||||
| WikiPathways | [+] 3 WikiPathways | + | ||||
| 1 | Integrated Pancreatic Cancer Pathway | |||||
| 2 | Neural Crest Differentiation | |||||
| 3 | Cell Cycle | |||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| Target QSAR Model | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | HDAC inhibitors: a 2013-2017 patent survey.Expert Opin Ther Pat. 2018 Apr 19:1-17. | |||||
| REF 2 | ClinicalTrials.gov (NCT03808870) A Safety and Pharmacokinetic Study of NBM-BMX Administered Orally to Asian Patients With Advanced Cancer. U.S. National Institutes of Health. | |||||
| REF 3 | Heterocyclic ketones as inhibitors of histone deacetylase. Bioorg Med Chem Lett. 2003 Nov 17;13(22):3909-13. | |||||
| REF 4 | New sulfurated derivatives of valproic acid with enhanced histone deacetylase inhibitory activity. Bioorg Med Chem Lett. 2008 Mar 15;18(6):1893-7. | |||||
| REF 5 | Structure-based optimization of phenylbutyrate-derived histone deacetylase inhibitors. J Med Chem. 2005 Aug 25;48(17):5530-5. | |||||
| REF 6 | Zn2+-chelating motif-tethered short-chain fatty acids as a novel class of histone deacetylase inhibitors. J Med Chem. 2004 Jan 15;47(2):467-74. | |||||
| REF 7 | Inhibitors of human histone deacetylase: synthesis and enzyme and cellular activity of straight chain hydroxamates. J Med Chem. 2002 Feb 14;45(4):753-7. | |||||
| REF 8 | Novel inhibitors of human histone deacetylases: design, synthesis, enzyme inhibition, and cancer cell growth inhibition of SAHA-based non-hydroxama... J Med Chem. 2005 Feb 24;48(4):1019-32. | |||||
| REF 9 | Chemical phylogenetics of histone deacetylases. Nat Chem Biol. 2010 Mar;6(3):238-243. | |||||
| REF 10 | Histone deacetylase inhibitors: from bench to clinic. J Med Chem. 2008 Mar 27;51(6):1505-29. | |||||
| REF 11 | Aromatic sulfide inhibitors of histone deacetylase based on arylsulfinyl-2,4-hexadienoic acid hydroxyamides. J Med Chem. 2006 Jan 26;49(2):800-5. | |||||
| REF 12 | Histone deacetylase inhibitors. J Med Chem. 2003 Nov 20;46(24):5097-116. | |||||
| REF 13 | Non-peptide macrocyclic histone deacetylase inhibitors. J Med Chem. 2009 Jan 22;52(2):456-68. | |||||
| REF 14 | Three new cyclostellettamines, which inhibit histone deacetylase, from a marine sponge of the genus Xestospongia. Bioorg Med Chem Lett. 2004 May 17;14(10):2617-20. | |||||
| REF 15 | Selective inhibition of histone deacetylases sensitizes malignant cells to death receptor ligands. Mol Cancer Ther. 2010 Jan;9(1):246-56. | |||||
| REF 16 | Mercaptoamide-based non-hydroxamic acid type histone deacetylase inhibitors. Bioorg Med Chem Lett. 2005 Apr 15;15(8):1969-72. | |||||
| REF 17 | Design and evaluation of 'Linkerless' hydroxamic acids as selective HDAC8 inhibitors. Bioorg Med Chem Lett. 2007 May 15;17(10):2874-8. | |||||
| REF 18 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 2619). | |||||
| REF 19 | N-Hydroxy-(4-oxime)-cinnamide: a versatile scaffold for the synthesis of novel histone deacetylase [correction of deacetilase] (HDAC) inhibitors. Bioorg Med Chem Lett. 2009 Apr 15;19(8):2346-9. | |||||
| REF 20 | Structural analysis of histone deacetylase 8 mutants associated with Cornelia de Lange Syndrome spectrum disorders. J Struct Biol. 2021 Mar;213(1):107681. | |||||
| REF 21 | Characterization of Histone Deacetylase 8 (HDAC8) Selective Inhibition Reveals Specific Active Site Structural and Functional Determinants. J Med Chem. 2018 Nov 21;61(22):10000-10016. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

