Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T60529
(Former ID: TTDS00040)
|
|||||
| Target Name |
Prostaglandin G/H synthase 1 (COX-1)
|
|||||
| Synonyms |
Prostaglandin-endoperoxide synthase 1; Prostaglandin H2 synthase 1; PHS 1; PGHS-1; PGH synthase 1; Cyclooxygenase-1; COX1; COX-1
Click to Show/Hide
|
|||||
| Gene Name |
PTGS1
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 12 Target-related Diseases | + | ||||
| 1 | Eye anterior segment structural developmental anomaly [ICD-11: LA11] | |||||
| 2 | Female pelvic pain [ICD-11: GA34] | |||||
| 3 | Hyper-lipoproteinaemia [ICD-11: 5C80] | |||||
| 4 | Indeterminate colitis [ICD-11: DD72] | |||||
| 5 | Nutritional deficiency [ICD-11: 5B50-5B71] | |||||
| 6 | Osteoarthritis [ICD-11: FA00-FA05] | |||||
| 7 | Pain [ICD-11: MG30-MG3Z] | |||||
| 8 | Postoperative inflammation [ICD-11: 1A00-CA43] | |||||
| 9 | Rheumatoid arthritis [ICD-11: FA20] | |||||
| 10 | Seborrhoeic dermatitis [ICD-11: EA81] | |||||
| 11 | Tuberculosis [ICD-11: 1B10-1B12] | |||||
| 12 | Ulcerative colitis [ICD-11: DD71] | |||||
| Function |
Converts arachidonate to prostaglandin H2 (PGH2), a committed step in prostanoid synthesis. Involved in the constitutive production of prostanoids in particular in the stomach and platelets. In gastric epithelial cells, it is a key step in the generation of prostaglandins, such as prostaglandin E2 (PGE2), which plays an important role in cytoprotection. In platelets, it is involved in the generation of thromboxane A2 (TXA2), which promotes platelet activation and aggregation, vasoconstriction and proliferation of vascular smooth muscle cells.
Click to Show/Hide
|
|||||
| BioChemical Class |
Paired donor oxygen oxidoreductase
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 1.14.99.1
|
|||||
| Sequence |
MSRSLLLWFLLFLLLLPPLPVLLADPGAPTPVNPCCYYPCQHQGICVRFGLDRYQCDCTR
TGYSGPNCTIPGLWTWLRNSLRPSPSFTHFLLTHGRWFWEFVNATFIREMLMRLVLTVRS NLIPSPPTYNSAHDYISWESFSNVSYYTRILPSVPKDCPTPMGTKGKKQLPDAQLLARRF LLRRKFIPDPQGTNLMFAFFAQHFTHQFFKTSGKMGPGFTKALGHGVDLGHIYGDNLERQ YQLRLFKDGKLKYQVLDGEMYPPSVEEAPVLMHYPRGIPPQSQMAVGQEVFGLLPGLMLY ATLWLREHNRVCDLLKAEHPTWGDEQLFQTTRLILIGETIKIVIEEYVQQLSGYFLQLKF DPELLFGVQFQYRNRIAMEFNHLYHWHPLMPDSFKVGSQEYSYEQFLFNTSMLVDYGVEA LVDAFSRQIAGRIGGGRNMDHHILHVAVDVIRESREMRLQPFNEYRKRFGMKPYTSFQEL VGEKEMAAELEELYGDIDALEFYPGLLLEKCHPNSIFGESMIEIGAPFSLKGLLGNPICS PEYWKPSTFGGEVGFNIVKTATLKKLVCLNTKTCPYVSFRVPDASQDDGPAVERPSTEL Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | PDB | ||||
| ADReCS ID | BADD_A05962 | |||||
| HIT2.0 ID | T00CXT | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 13 Approved Drugs | + | ||||
| 1 | Aminosalicylic Acid | Drug Info | Approved | Pulmonary and extrapulmonary tuberculosis | [2] | |
| 2 | Balsalazide | Drug Info | Approved | Inflammatory bowel disease | [4] | |
| 3 | Bromfenac | Drug Info | Approved | Postoperative inflammation | [5], [6] | |
| 4 | Eicosapentaenoic acid/docosa-hexaenoic acid | Drug Info | Approved | Hypertriglyceridemia | [7] | |
| 5 | FENBUFEN | Drug Info | Approved | Arthritis | [2] | |
| 6 | Flufenamic Acid | Drug Info | Approved | Dysmenorrhea | [2], [8] | |
| 7 | Gamma-Homolinolenic acid | Drug Info | Approved | Malnutrition | [9] | |
| 8 | Meclofenamate Sodium | Drug Info | Approved | Arthritis | [2] | |
| 9 | Mesalazine | Drug Info | Approved | Ulcerative colitis | [10], [11] | |
| 10 | Naproxen | Drug Info | Approved | Osteoarthritis | [2] | |
| 11 | Piroxicam | Drug Info | Approved | Pain | [12], [13] | |
| 12 | Salicyclic acid | Drug Info | Approved | Seborrhoeic dermatitis | [2], [14], [15] | |
| 13 | Suprofen | Drug Info | Approved | Miosis | [2], [16], [17] | |
| Clinical Trial Drug(s) | [+] 5 Clinical Trial Drugs | + | ||||
| 1 | (S)-FLURBIPROFEN | Drug Info | Preregistration | Myalgia | [18] | |
| 2 | Curcumin | Drug Info | Phase 3 | Solid tumour/cancer | [20], [21] | |
| 3 | ThermoProfen | Drug Info | Phase 3 | Pain | [22] | |
| 4 | EPICATECHIN | Drug Info | Phase 1/2 | Duchenne dystrophy | [23] | |
| 5 | EXO-230 | Drug Info | Phase 1/2 | Diabetic neuropathy | [24] | |
| Discontinued Drug(s) | [+] 6 Discontinued Drugs | + | ||||
| 1 | INDOPROFEN | Drug Info | Withdrawn from market | Gout | [2] | |
| 2 | Metamizole | Drug Info | Withdrawn from market | Pain | [2] | |
| 3 | Phenacetin | Drug Info | Withdrawn from market | Analgesia | [25], [26] | |
| 4 | CRx-401 | Drug Info | Discontinued in Phase 2 | Type-2 diabetes | [27] | |
| 5 | TEBUFELONE | Drug Info | Discontinued in Phase 2 | Pain | [28] | |
| 6 | ATLIPROFEN METHYL ESTER | Drug Info | Terminated | Inflammation | [29] | |
| Mode of Action | [+] 3 Modes of Action | + | ||||
| Modulator | [+] 5 Modulator drugs | + | ||||
| 1 | Aminosalicylic Acid | Drug Info | [30] | |||
| 2 | Meclofenamate Sodium | Drug Info | [39] | |||
| 3 | Piroxicam | Drug Info | [30] | |||
| 4 | ATLIPROFEN METHYL ESTER | Drug Info | [30] | |||
| 5 | Nitroflurbiprofen | Drug Info | [85], [86], [87] | |||
| Inhibitor | [+] 115 Inhibitor drugs | + | ||||
| 1 | Balsalazide | Drug Info | [31] | |||
| 2 | Bromfenac | Drug Info | [32] | |||
| 3 | Eicosapentaenoic acid/docosa-hexaenoic acid | Drug Info | [33] | |||
| 4 | FENBUFEN | Drug Info | [34] | |||
| 5 | Flufenamic Acid | Drug Info | [35] | |||
| 6 | Gamma-Homolinolenic acid | Drug Info | [36], [37], [38] | |||
| 7 | Mesalazine | Drug Info | [1] | |||
| 8 | Naproxen | Drug Info | [40] | |||
| 9 | Salicyclic acid | Drug Info | [41] | |||
| 10 | Suprofen | Drug Info | [42] | |||
| 11 | IMRECOXIB | Drug Info | [43] | |||
| 12 | (S)-FLURBIPROFEN | Drug Info | [44] | |||
| 13 | Curcumin | Drug Info | [45] | |||
| 14 | ThermoProfen | Drug Info | [46] | |||
| 15 | EPICATECHIN | Drug Info | [47] | |||
| 16 | Carbamate derivative 2 | Drug Info | [49] | |||
| 17 | INDOPROFEN | Drug Info | [44] | |||
| 18 | Metamizole | Drug Info | [50] | |||
| 19 | Phenacetin | Drug Info | [51] | |||
| 20 | CRx-401 | Drug Info | [52] | |||
| 21 | TEBUFELONE | Drug Info | [53] | |||
| 22 | SC-58451 | Drug Info | [54] | |||
| 23 | (-)-3-O-acetylspectaline | Drug Info | [55] | |||
| 24 | (11H-Dibenzo[b,e][1,4]dioxepin-2-yl)-acetic acid | Drug Info | [56] | |||
| 25 | (11H-Dibenzo[b,e][1,4]dioxepin-8-yl)-acetic acid | Drug Info | [56] | |||
| 26 | (3-Chloro-4-Propoxy-Phenyl)-Acetic Acid | Drug Info | [57] | |||
| 27 | (R)-2-(4-Isobutyl-phenyl)-N-phenyl-propionamide | Drug Info | [44] | |||
| 28 | (Z)-2'-des-methyl sulindac sulfide | Drug Info | [58] | |||
| 29 | 1,2-dihydro-3-(2,3,4-trimethoxyphenyl)naphthalene | Drug Info | [59] | |||
| 30 | 1-(4-(methylsulfonyl)phenyl)-3-p-tolylurea | Drug Info | [60] | |||
| 31 | 1-(4-(methylsulfonyl)phenyl)-3-phenylurea | Drug Info | [60] | |||
| 32 | 1-(4-aminosulfonylphenyl)-2-(4-pyridyl)acetylene | Drug Info | [61] | |||
| 33 | 2'-epi-guianin | Drug Info | [62] | |||
| 34 | 2,4'-Dimethoxy-5,3'-di-(2-propenyl)-biphenyl | Drug Info | [63] | |||
| 35 | 2-(1,1'-Biphenyl-4-Yl)Propanoic Acid | Drug Info | [57], [64] | |||
| 36 | 2-(2,3,4-trimethoxyphenyl)-1H-indene | Drug Info | [59] | |||
| 37 | 2-(2-(2,6-dimethylphenylamino)phenyl)acetic acid | Drug Info | [65] | |||
| 38 | 2-(2-methoxyphenyl)-1H-indene | Drug Info | [59] | |||
| 39 | 2-(2-Methylpropanoyl)-1,3,5-benzenetriol | Drug Info | [66] | |||
| 40 | 2-(3'-Allyl-biphenyl-4-yl)-propionic acid | Drug Info | [67] | |||
| 41 | 2-(3'-Ethyl-biphenyl-4-yl)-propionic acid | Drug Info | [67] | |||
| 42 | 2-(3'-Ethylsulfanyl-biphenyl-4-yl)-propionic acid | Drug Info | [67] | |||
| 43 | 2-(3-Phenyl-propyl)-1,2-dihydro-indazol-3-one | Drug Info | [68] | |||
| 44 | 2-(N-(2-Ffuorophenyl)pyrrol-3-yl) acetic acid | Drug Info | [69] | |||
| 45 | 2-(N-(2-fluorophenyl)pyrrol-2-yl) acetic acid | Drug Info | [69] | |||
| 46 | 2-(p-Methylsulfonylbenzoyl)furan | Drug Info | [70] | |||
| 47 | 2-Benzyl-1,2-dihydro-indazol-3-one | Drug Info | [68] | |||
| 48 | 2-Bromoacetyl Group | Drug Info | [71] | |||
| 49 | 2-Furan-2-ylmethyl-1,2-dihydro-indazol-3-one | Drug Info | [68] | |||
| 50 | 2-Phenethyl-1,2-dihydro-indazol-3-one | Drug Info | [68] | |||
| 51 | 2-Phenyl-1,2-dihydro-indazol-3-one | Drug Info | [68] | |||
| 52 | 2-[4-(1H-Indol-5-yl)-phenyl]-propionic acid | Drug Info | [67] | |||
| 53 | 3-(4-Methanesulfonyl-phenyl)-1-phenyl-propynone | Drug Info | [72] | |||
| 54 | 4'-Methoxy-5,3'-dipropyl-biphenyl-2ol | Drug Info | [63] | |||
| 55 | 4,5-Bis(4-chlorophenyl)-1,2-selenazole | Drug Info | [73] | |||
| 56 | 4,5-Bis(4-chlorophenyl)isothiazole | Drug Info | [74] | |||
| 57 | 4,5-Bis(4-methoxyphenyl)-1,2-selenazole | Drug Info | [73] | |||
| 58 | 4,5-Bis(4-methoxyphenyl)-3H-1,2-dithiole-3-thione | Drug Info | [74] | |||
| 59 | 4,5-Bis(4-methoxyphenyl)isothiazole | Drug Info | [74] | |||
| 60 | 4-(4-Chlorophenyl)-5-(4-methoxyphenyl)isothiazole | Drug Info | [74] | |||
| 61 | 4-(4-Chlorophenyl)-5-p-tolyl-1,2-selenazole | Drug Info | [73] | |||
| 62 | 4-(4-Chlorophenyl)-5-p-tolyl-3H-1,2-dithiol-3-one | Drug Info | [74] | |||
| 63 | 4-(4-Chlorophenyl)-5-p-tolylisothiazole | Drug Info | [74] | |||
| 64 | 4-(5-(4-Hydroxyphenyl)isothiazol-4-yl)phenol | Drug Info | [74] | |||
| 65 | 4-amino-N-(4-chlorophenyl)benzenesulfonamide | Drug Info | [75] | |||
| 66 | 4-amino-N-p-tolylbenzenesulfonamide | Drug Info | [75] | |||
| 67 | 5,3'-Dipropyl-biphenyl-2,4'-diol | Drug Info | [63] | |||
| 68 | 5-(2-1H-indenyl)-1,3-benzodioxole | Drug Info | [59] | |||
| 69 | 5-(2-Imidazol-1-yl-ethyl)-7,8-dihydro-quinoline | Drug Info | [76] | |||
| 70 | 5-(4-Chlorophenyl)-4-(4-methoxyphenyl)isothiazole | Drug Info | [74] | |||
| 71 | 5-(4-Chlorophenyl)-4-p-tolyl-1,2-selenazole | Drug Info | [73] | |||
| 72 | 5-(4-Chlorophenyl)-4-p-tolyl-3H-1,2-dithiol-3-one | Drug Info | [74] | |||
| 73 | 5-(4-Chlorophenyl)-4-p-tolylisothiazole | Drug Info | [74] | |||
| 74 | 5-(4-Methoxyphenyl)-4-p-tolyl-1,2-selenazole | Drug Info | [73] | |||
| 75 | 5-(4-Methoxyphenyl)-4-p-tolylisothiazole | Drug Info | [74] | |||
| 76 | 5-Ethyl-3,4-diphenyl-isoxazole | Drug Info | [77] | |||
| 77 | 5-Methyl-3,4-diphenyl-isoxazole | Drug Info | [77] | |||
| 78 | 5-Phenyl-pentanoic acid benzyl-hydroxy-amide | Drug Info | [78] | |||
| 79 | Acetic acid 2-hept-2-ynylsulfanyl-phenyl ester | Drug Info | [79] | |||
| 80 | Acetic acid 2-hept-3-ynylsulfanyl-phenyl ester | Drug Info | [79] | |||
| 81 | Acetic acid 2-heptylselanyl-phenyl ester | Drug Info | [79] | |||
| 82 | Acetic acid 2-hex-2-ynylsulfanyl-phenyl ester | Drug Info | [79] | |||
| 83 | Acetic acid 2-hexylsulfanyl-phenyl ester | Drug Info | [79] | |||
| 84 | Acetic acid 2-pentylsulfanyl-phenyl ester | Drug Info | [79] | |||
| 85 | Acetic Acid Salicyloyl-Amino-Ester | Drug Info | [57] | |||
| 86 | Alpha-D-Mannose | Drug Info | [71] | |||
| 87 | Arachidonic Acid | Drug Info | [71] | |||
| 88 | B-Octylglucoside | Drug Info | [71] | |||
| 89 | Beta-D-Glucose | Drug Info | [71] | |||
| 90 | Beta-D-Mannose | Drug Info | [71] | |||
| 91 | DEMETHOXYCURCUMIN | Drug Info | [45] | |||
| 92 | FR122047 | Drug Info | [80] | |||
| 93 | HONOKIOL | Drug Info | [63] | |||
| 94 | Hyperforin | Drug Info | [81] | |||
| 95 | IODOINDOMETHACIN | Drug Info | [64] | |||
| 96 | IODOSUPROFEN | Drug Info | [64] | |||
| 97 | METHYLHONOKIOL | Drug Info | [63] | |||
| 98 | N-(1H-indazol-5-yl)acetamide | Drug Info | [82] | |||
| 99 | N-(3-(phenylthio)pyridin-4-yl)methanesulfonamide | Drug Info | [83] | |||
| 100 | N-(3-phenoxy-4-pyridinyl)ethanesulfonamide | Drug Info | [84] | |||
| 101 | N-(3-phenoxy-4-pyridinyl)propanesulfonamide | Drug Info | [84] | |||
| 102 | N-(3-phenylamino-4-pyridinyl)methanesulfonamide | Drug Info | [83] | |||
| 103 | O-Acetylserine | Drug Info | [71] | |||
| 104 | Oxametacin | Drug Info | [88] | |||
| 105 | P-(2'-Iodo-5'-Thenoyl)Hydrotropic Acid | Drug Info | [57] | |||
| 106 | Phenazone | Drug Info | [89] | |||
| 107 | PHENIDONE | Drug Info | [68] | |||
| 108 | Prifelone | Drug Info | [90] | |||
| 109 | Primary alcohol metabolite of celecoxib | Drug Info | [91] | |||
| 110 | Protoporphyrin Ix Containing Co | Drug Info | [71] | |||
| 111 | RESORCINOL | Drug Info | [47] | |||
| 112 | Resveratrol Potassium3-Sulfate | Drug Info | [92] | |||
| 113 | Resveratrol Potassium4,-Sulfate | Drug Info | [92] | |||
| 114 | SC-560 | Drug Info | [93] | |||
| 115 | TRL-382 | Drug Info | [94] | |||
| Agonist | [+] 1 Agonist drugs | + | ||||
| 1 | EXO-230 | Drug Info | [48] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
| Protein Name | Pfam ID | Percentage of Identity (%) | E value |
|---|---|---|---|
| Neurexin-2 (NRXN2) | 38.095 (24/63) | 6.96E-05 |
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Arachidonic acid metabolism | hsa00590 | Affiliated Target |
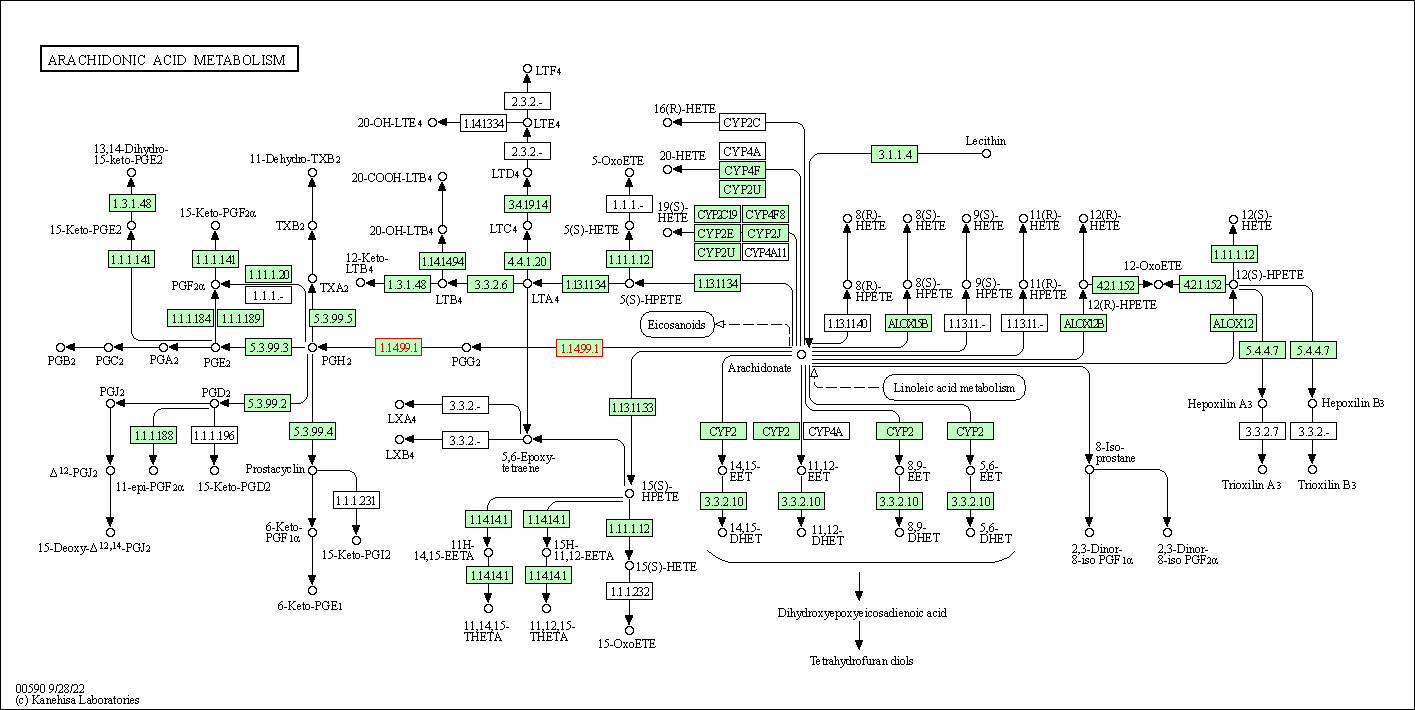
|
| Class: Metabolism => Lipid metabolism | Pathway Hierarchy | ||
| Platelet activation | hsa04611 | Affiliated Target |
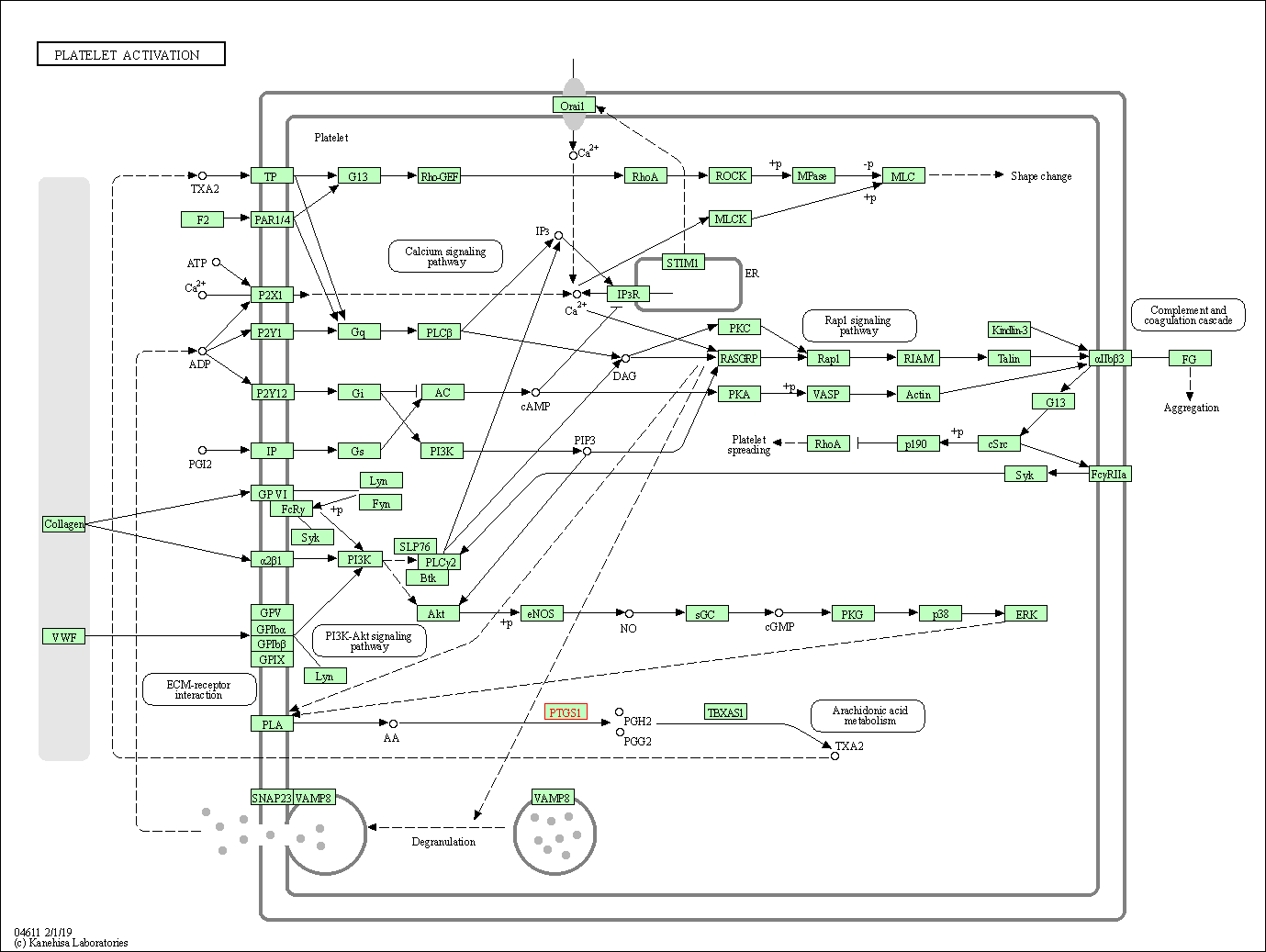
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Serotonergic synapse | hsa04726 | Affiliated Target |
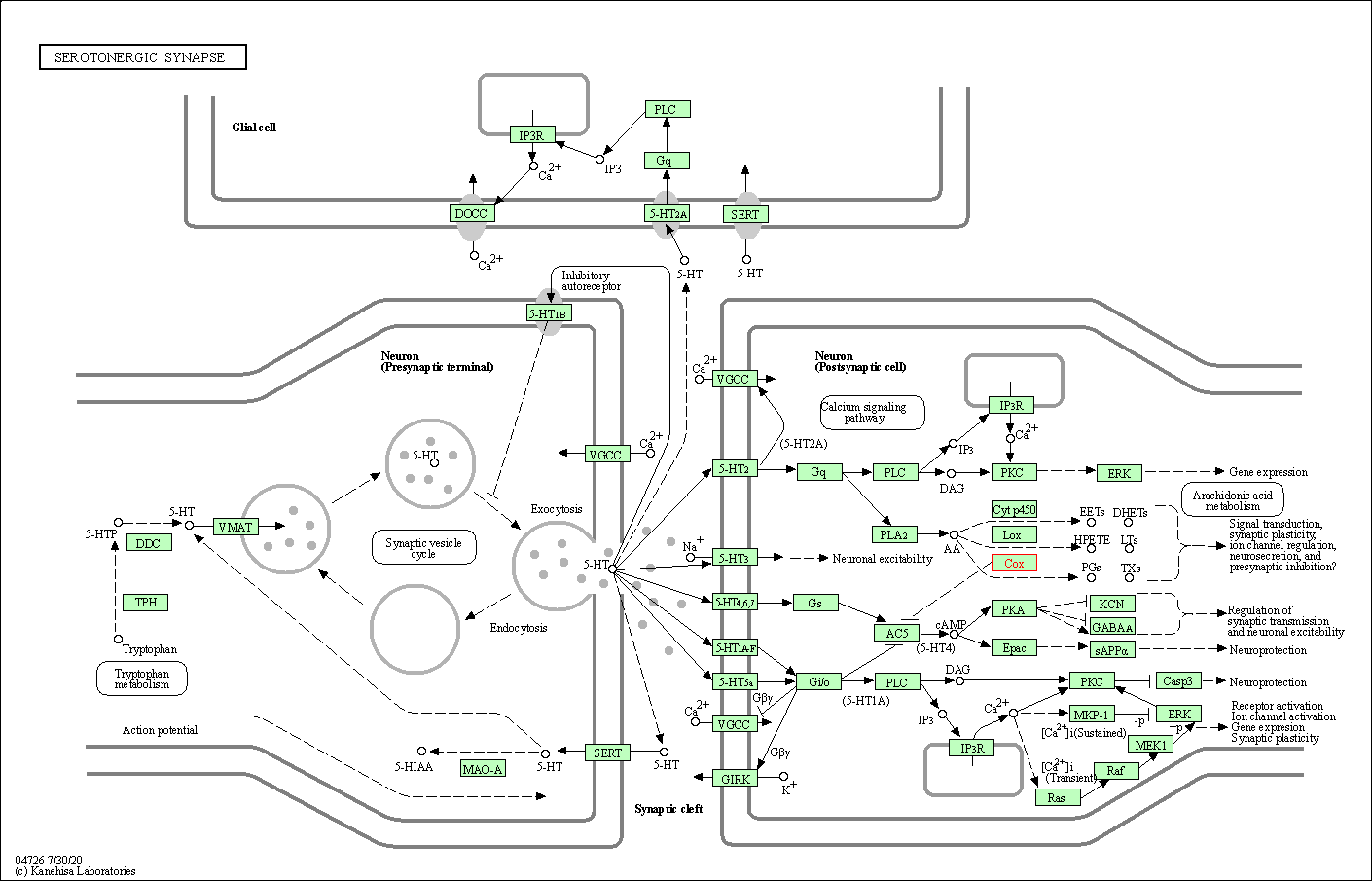
|
| Class: Organismal Systems => Nervous system | Pathway Hierarchy | ||
| Regulation of lipolysis in adipocytes | hsa04923 | Affiliated Target |
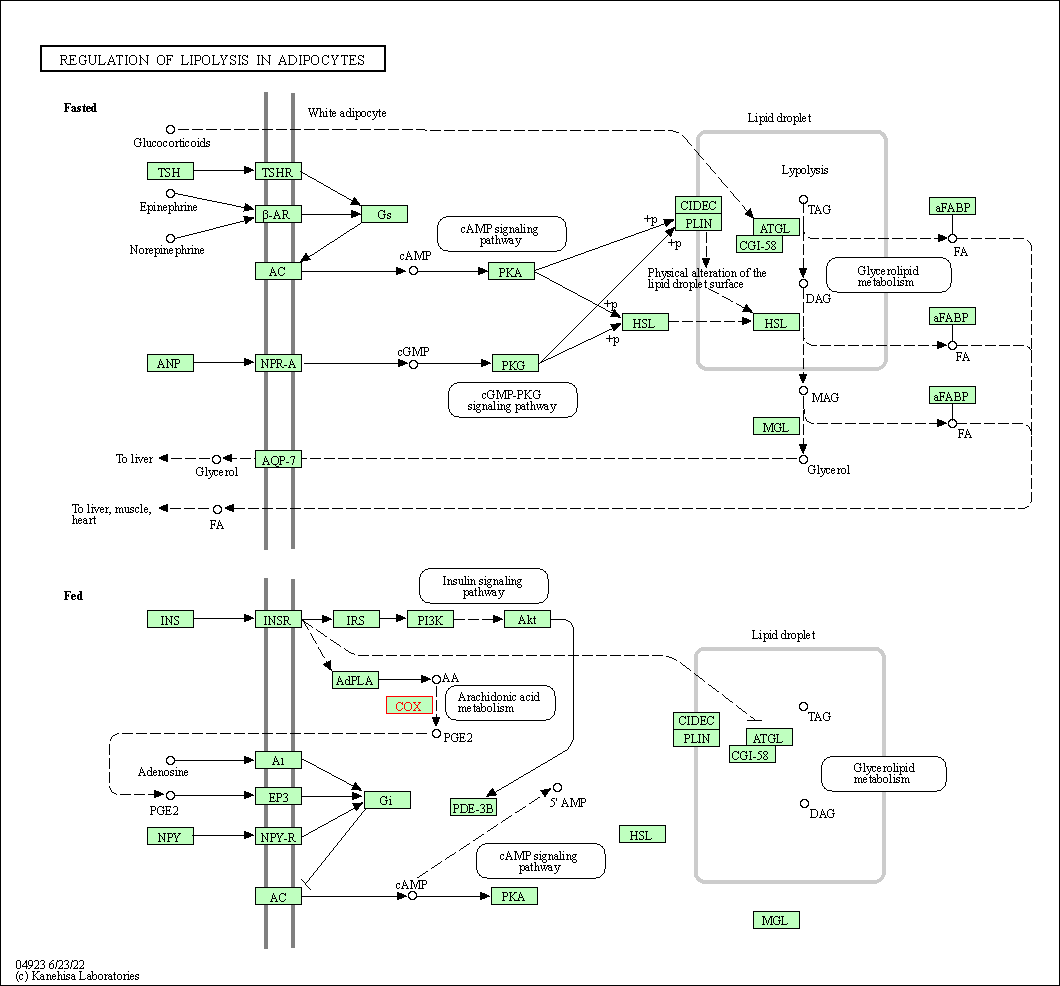
|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Degree | 8 | Degree centrality | 8.59E-04 | Betweenness centrality | 4.06E-05 |
|---|---|---|---|---|---|
| Closeness centrality | 1.92E-01 | Radiality | 1.33E+01 | Clustering coefficient | 2.14E-01 |
| Neighborhood connectivity | 7.13E+00 | Topological coefficient | 2.01E-01 | Eccentricity | 12 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) | ||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| BioCyc | [+] 1 BioCyc Pathways | + | ||||
| 1 | C20 prostanoid biosynthesis | |||||
| KEGG Pathway | [+] 4 KEGG Pathways | + | ||||
| 1 | Arachidonic acid metabolism | |||||
| 2 | Metabolic pathways | |||||
| 3 | Platelet activation | |||||
| 4 | Serotonergic synapse | |||||
| NetPath Pathway | [+] 1 NetPath Pathways | + | ||||
| 1 | TGF_beta_Receptor Signaling Pathway | |||||
| Panther Pathway | [+] 1 Panther Pathways | + | ||||
| 1 | Inflammation mediated by chemokine and cytokine signaling pathway | |||||
| Pathwhiz Pathway | [+] 1 Pathwhiz Pathways | + | ||||
| 1 | Arachidonic Acid Metabolism | |||||
| WikiPathways | [+] 5 WikiPathways | + | ||||
| 1 | Prostaglandin Synthesis and Regulation | |||||
| 2 | Arachidonic acid metabolism | |||||
| 3 | Phase 1 - Functionalization of compounds | |||||
| 4 | Eicosanoid Synthesis | |||||
| 5 | Selenium Micronutrient Network | |||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| Target QSAR Model | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | How many drug targets are there Nat Rev Drug Discov. 2006 Dec;5(12):993-6. | |||||
| REF 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||||
| REF 3 | Drug information of Salsalate, 2008. eduDrugs. | |||||
| REF 4 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 077806. | |||||
| REF 5 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7131). | |||||
| REF 6 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 021664. | |||||
| REF 7 | FDA approves EPANOVA for the treatment of adults with severe hypertriglyceridemia | |||||
| REF 8 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2447). | |||||
| REF 9 | Treatment of rheumatoid arthritis with gammalinolenic acid. Ann Intern Med. 1993 Nov 1;119(9):867-73. | |||||
| REF 10 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4655). | |||||
| REF 11 | BiDil: assessing a race-based pharmaceutical. Ann Fam Med. 2006 Nov-Dec;4(6):556-60. | |||||
| REF 12 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7273). | |||||
| REF 13 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 073535. | |||||
| REF 14 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4306). | |||||
| REF 15 | Drug information of Salicyclic acid, 2008. eduDrugs. | |||||
| REF 16 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7298). | |||||
| REF 17 | Drug information of Suprofen, 2008. eduDrugs. | |||||
| REF 18 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800025057) | |||||
| REF 19 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 20 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7000). | |||||
| REF 21 | Nanocurcumin: a promising therapeutic advancement over native curcumin. Crit Rev Ther Drug Carrier Syst. 2013;30(4):331-68. | |||||
| REF 22 | ClinicalTrials.gov (NCT00488267) Efficacy of ThermoProfen in Patients With Mild to Moderate Pain Associated With Osteoarthritis of the Knee. U.S. National Institutes of Health. | |||||
| REF 23 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 24 | ClinicalTrials.gov (NCT00544934) Multiple Dose Trial of Anti-glycation Agent GLY-230 in Healthy and Diabetic Subjects. U.S. National Institutes of Health. | |||||
| REF 25 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7402). | |||||
| REF 26 | An epidemiologic study of abuse of analgesic drugs. Effects of phenacetin and salicylate on mortality and cardiovascular morbidity (1968 to 1987)Dubach UC1, Rosner B, St rmer T.Author information1Department of Internal Medicine, Kantonsspital, Basel, Switzerland.AbstractBACKGROUND: Phenacetin abuse is known to produce kidney disease; salicylate use is supposed to prevent cardiovascular disease. We conducted a prospective, longitudinal epidemiologic study to examine the effects of these drugs on cause-specific mortality and on cardiovascular morbidity.METHODS: In 1968 we evaluated a study group of 623 healthy women 30 to 49 years old who had evidence of a regular intake of phenacetin, as measured by urinary excretion of its metabolites, and a matched control group of 621 women. Salicylate excretion was also measured. All subjects were examined over a period of 20 years.RESULTS: Life-table analyses of mortality during the 20 years, with adjustment for the year of birth, cigarette smoking, and length of follow-up, revealed significant differences between the groups in overall mortality (study group vs. control group, 74 vs. 27 deaths; relative risk, 2.2; 95 percent confidence interval, 1.5 to 3.3), deaths due to urologic or renal disease (relative risk, 16.1; 95 percent confidence interval, 3.9 to 66.1), deaths due to cancer (relative risk, 1.9; 95 percent confidence interval, 1.1 to 3.3), and deaths due to cardiovascular disease (relative risk, 2.9; 95 percent confidence interval, 1.5 to 5.5). The relative risk of cardiovascular disease (fatal or nonfatal myocardial infarction, heart failure, or stroke) was 1.8, and the 95 percent confidence interval 1.3 to 2.6. The odds ratio for the incidence of hypertension was 1.6, and the 95 percent confidence interval 1.2 to 2.1. The effects of phenacetin on morbidity and mortality, with adjustment for base-line salicylate excretion, were similar. In contrast, salicylate use had no effect on either mortality or morbidity.CONCLUSIONS: Regular use of analgesic drugs containing phenacetin is associated with an increased risk of hypertension and mortality and morbidity due to cardiovascular disease, as well as an increased riskof mortality due to cancer and urologic or renal disease. The use of salicylates carries no such risk.Comment inThe risks of phenacetin use. [N Engl J Med. 1991]PMID: 1984193 [PubMed - indexed for MEDLINE] Free full textSharePublication Types, MeSH Terms, SubstancesPublication TypesResearch Support, Non-U.S. Gov'tMeSH TermsAdultAgedAspirin/adverse effects*Cardiovascular Diseases/epidemiology*Cardiovascular Diseases/mortalityDose-Response Relationship, DrugFemaleHumansHypertension/epidemiologyKidney Diseases/mortalityLongitudinal StudiesMiddle AgedMortality*Neoplasms/mortalityPhenacetin/adverse effects*Prospective StudiesRiskSubstance-Related Disorders*Urologic Diseases/mortalitySubstancesPhenacetinAspirinLinkOut - more resourcesFull Text SourcesAtypon - PDFAtyponOvid Technologies, Inc.MedicalDrug Abuse - MedlinePlus Health InformationMiscellaneousACETYLSALICYLIC ACID - Hazardous Substances Data BankPHENACETIN - Hazardous Substances Data BankPubMed Commons home N Engl J Med. 1991 Jan 17;324(3):155-60. | |||||
| REF 27 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800018957) | |||||
| REF 28 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002346) | |||||
| REF 29 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800000964) | |||||
| REF 30 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||||
| REF 31 | Drugs, their targets and the nature and number of drug targets. Nat Rev Drug Discov. 2006 Oct;5(10):821-34. | |||||
| REF 32 | Comparison of cyclooxygenase inhibitory activity and ocular anti-inflammatory effects of ketorolac tromethamine and bromfenac sodium. Curr Med Res Opin. 2006 Jun;22(6):1133-40. | |||||
| REF 33 | Cox-2 inhibitory effects of naturally occurring and modified fatty acids. J Nat Prod. 2001 Jun;64(6):745-9. | |||||
| REF 34 | Fenbufen based 3-[5-(substituted aryl)-1,3,4-oxadiazol-2-yl]-1-(biphenyl-4-yl)propan-1-ones as safer antiinflammatory and analgesic agents. Eur J Med Chem. 2009 Sep;44(9):3798-804. | |||||
| REF 35 | Ouellet M, Percival MD: Effect of inhibitor time-dependency on selectivity towards cyclooxygenase isoforms. Biochem J. 1995 Feb 15;306 ( Pt 1):247-51. | |||||
| REF 36 | Differential metabolism of dihomo-gamma-linolenic acid and arachidonic acid by cyclo-oxygenase-1 and cyclo-oxygenase-2: implications for cellular synthesis of prostaglandin E1 and prostaglandin E2. Biochem J. 2002 Jul 15;365(Pt 2):489-96. | |||||
| REF 37 | Structure of eicosapentaenoic and linoleic acids in the cyclooxygenase site of prostaglandin endoperoxide H synthase-1. J Biol Chem. 2001 Oct 5;276(40):37547-55. | |||||
| REF 38 | Mutational and X-ray crystallographic analysis of the interaction of dihomo-gamma -linolenic acid with prostaglandin endoperoxide H synthases. J Biol Chem. 2001 Mar 30;276(13):10358-65. | |||||
| REF 39 | Role of COX-2-derived metabolites in regulation of the renal hemodynamic response to norepinephrine. Am J Physiol Renal Physiol. 2001 Nov;281(5):F975-82. | |||||
| REF 40 | Comparative inhibitory activity of rofecoxib, meloxicam, diclofenac, ibuprofen, and naproxen on COX-2 versus COX-1 in healthy volunteers. J Clin Pharmacol. 2000 Oct;40(10):1109-20. | |||||
| REF 41 | The C50T polymorphism of the cyclooxygenase-1 gene and the risk of thrombotic events during low-dose therapy with acetyl salicylic acid. Thromb Haemost. 2008 Jul;100(1):70-5. | |||||
| REF 42 | Differential binding mode of diverse cyclooxygenase inhibitors. J Mol Graph Model. 2002 Mar;20(5):359-71. | |||||
| REF 43 | Synthesis and anti-inflammatory activity of the major metabolites of imrecoxib. Bioorg Med Chem Lett. 2009 Apr 15;19(8):2270-2. | |||||
| REF 44 | 2-Arylpropionic CXC chemokine receptor 1 (CXCR1) ligands as novel noncompetitive CXCL8 inhibitors. J Med Chem. 2005 Jun 30;48(13):4312-31. | |||||
| REF 45 | Design, synthesis, biological evaluation and molecular docking of curcumin analogues as antioxidant, cyclooxygenase inhibitory and anti-inflammator... Bioorg Med Chem Lett. 2005 Apr 1;15(7):1793-7. | |||||
| REF 46 | Topical NSAID therapy for musculoskeletal pain. Pain Med. 2010 Apr;11(4):535-49. | |||||
| REF 47 | Mechanism-based inactivation of COX-1 by red wine m-hydroquinones: a structure-activity relationship study. J Nat Prod. 2004 Nov;67(11):1777-82. | |||||
| REF 48 | Inhibiting Amadori-modified albumin formation improves biomarkers of podocyte damage in diabetic rats. Physiol Rep. 2013 September; 1(4): e00083. | |||||
| REF 49 | Fatty acid amide hydrolase inhibitors: a patent review (2009-2014).Expert Opin Ther Pat. 2015;25(11):1247-66. | |||||
| REF 50 | Mechanisms of action of paracetamol and related analgesics. Inflammopharmacology. 2003;11(4):401-13. | |||||
| REF 51 | Direct toxicity of nonsteroidal antiinflammatory drugs for renal medullary cells. Proc Natl Acad Sci U S A. 2001 Apr 24;98(9):5317-22. | |||||
| REF 52 | ClinicalTrials.gov (NCT00506298) Study of CRx-401 on Glucose Levels in Subjects With Type II Diabetes. U.S. National Institutes of Health. | |||||
| REF 53 | New cyclooxygenase-2/5-lipoxygenase inhibitors. 2. 7-tert-butyl-2,3-dihydro-3,3-dimethylbenzofuran derivatives as gastrointestinal safe antiinflamm... J Med Chem. 1998 Mar 26;41(7):1124-37. | |||||
| REF 54 | Novel 1,2-diarylcyclobutenes: Selective and orally active cox-2 inhibitors, Bioorg. Med. Chem. Lett. 6(22):2677-2682 (1996). | |||||
| REF 55 | Lipoperoxidation and cyclooxygenase enzyme inhibitory piperidine alkaloids from Cassia spectabilis green fruits. J Nat Prod. 2007 Dec;70(12):2026-8. | |||||
| REF 56 | Synthesis and antiinflammatory/analgesic activities of 11H-dibenzo[b, e,][1,4]dioxepinacetic acids. J Med Chem. 1986 Aug;29(8):1436-41. | |||||
| REF 57 | The Protein Data Bank. Nucleic Acids Res. 2000 Jan 1;28(1):235-42. | |||||
| REF 58 | The influence of double bond geometry in the inhibition of cyclooxygenases by sulindac derivatives. Bioorg Med Chem Lett. 2009 Jun 15;19(12):3271-4. | |||||
| REF 59 | 'Bridged' stilbene derivatives as selective cyclooxygenase-1 inhibitors. Bioorg Med Chem. 2007 Sep 15;15(18):6109-18. | |||||
| REF 60 | Design and synthesis of 1,3-diarylurea derivatives as selective cyclooxygenase (COX-2) inhibitors. Bioorg Med Chem Lett. 2008 Feb 15;18(4):1336-9. | |||||
| REF 61 | Synthesis and cyclooxygenase inhibitory activities of linear 1-(methanesulfonylphenyl or benzenesulfonamido)-2-(pyridyl)acetylene regioisomers. Bioorg Med Chem. 2008 Feb 15;16(4):1948-56. | |||||
| REF 62 | COX, LOX and platelet aggregation inhibitory properties of Lauraceae neolignans. Bioorg Med Chem Lett. 2009 Dec 15;19(24):6922-5. | |||||
| REF 63 | Design and synthesis of ten biphenyl-neolignan derivatives and their in vitro inhibitory potency against cyclooxygenase-1/2 activity and 5-lipoxyge... Bioorg Med Chem. 2009 Jul 1;17(13):4459-65. | |||||
| REF 64 | In silico search for multi-target anti-inflammatories in Chinese herbs and formulas. Bioorg Med Chem. 2010 Mar 15;18(6):2204-2218. | |||||
| REF 65 | Molecular determinants for the selective inhibition of cyclooxygenase-2 by lumiracoxib. J Biol Chem. 2007 Jun 1;282(22):16379-90. | |||||
| REF 66 | Anti-inflammatory acylphloroglucinol derivatives from Hops (Humulus lupulus). J Nat Prod. 2005 Oct;68(10):1545-8. | |||||
| REF 67 | Structure-based design of COX-2 selectivity into flurbiprofen. Bioorg Med Chem Lett. 1999 Feb 8;9(3):307-12. | |||||
| REF 68 | Indazolinones, a new series of redox-active 5-lipoxygenase inhibitors with built-in selectivity and oral activity. J Med Chem. 1991 Mar;34(3):1028-36. | |||||
| REF 69 | Synthesis and biological activity of new anti-inflammatory compounds containing the 1,4-benzodioxine and/or pyrrole system. Bioorg Med Chem. 2007 Jul 15;15(14):4876-90. | |||||
| REF 70 | Synthesis, anti-inflammatory activity, and in vitro antitumor effect of a novel class of cyclooxygenase inhibitors: 4-(aryloyl)phenyl methyl sulfones. J Med Chem. 2010 Sep 23;53(18):6560-71. | |||||
| REF 71 | How many drug targets are there Nat Rev Drug Discov. 2006 Dec;5(12):993-6. | |||||
| REF 72 | Synthesis and biological evaluation of 1,3-diphenylprop-2-yn-1-ones as dual inhibitors of cyclooxygenases and lipoxygenases. Bioorg Med Chem Lett. 2005 Nov 1;15(21):4842-5. | |||||
| REF 73 | Investigations concerning the COX/5-LOX inhibiting and hydroxyl radical scavenging potencies of novel 4,5-diaryl isoselenazoles. Eur J Med Chem. 2008 Jun;43(6):1152-9. | |||||
| REF 74 | Diaryl-dithiolanes and -isothiazoles: COX-1/COX-2 and 5-LOX-inhibitory, *OH scavenging and anti-adhesive activities. Bioorg Med Chem. 2009 Jan 15;17(2):558-68. | |||||
| REF 75 | Analgesic agents without gastric damage: design and synthesis of structurally simple benzenesulfonanilide-type cyclooxygenase-1-selective inhibitors. Bioorg Med Chem. 2007 Jan 15;15(2):1014-21. | |||||
| REF 76 | 1-imidazolyl(alkyl)-substituted di- and tetrahydroquinolines and analogues: syntheses and evaluation of dual inhibitors of thromboxane A(2) synthas... J Med Chem. 2000 May 4;43(9):1841-51. | |||||
| REF 77 | Novel synthesis of 3,4-diarylisoxazole analogues of valdecoxib: reversal cyclooxygenase-2 selectivity by sulfonamide group removal. J Med Chem. 2004 Sep 23;47(20):4881-90. | |||||
| REF 78 | Differential effects of a series of hydroxamic acid derivatives on 5-lipoxygenase and cyclooxygenase from neutrophils and 12-lipoxygenase from plat... J Med Chem. 1989 Aug;32(8):1836-42. | |||||
| REF 79 | Covalent modification of cyclooxygenase-2 (COX-2) by 2-acetoxyphenyl alkyl sulfides, a new class of selective COX-2 inactivators. J Med Chem. 1998 Nov 19;41(24):4800-18. | |||||
| REF 80 | The analgesic effect profile of FR122047, a selective cyclooxygenase-1 inhibitor, in chemical nociceptive models. Eur J Pharmacol. 2000 Mar 10;391(1-2):49-54. | |||||
| REF 81 | Hyperforin is a dual inhibitor of cyclooxygenase-1 and 5-lipoxygenase. Biochem Pharmacol. 2002 Dec 15;64(12):1767-75. | |||||
| REF 82 | New analgesics synthetically derived from the paracetamol metabolite N-(4-hydroxyphenyl)-(5Z,8Z,11Z,14Z)-icosatetra-5,8,11,14-enamide. J Med Chem. 2008 Dec 25;51(24):7800-5. | |||||
| REF 83 | Pyridine analogues of nimesulide: design, synthesis, and in vitro and in vivo pharmacological evaluation as promising cyclooxygenase 1 and 2 inhibi... J Med Chem. 2009 Oct 8;52(19):5864-71. | |||||
| REF 84 | Design, synthesis, and pharmacological evaluation of pyridinic analogues of nimesulide as cyclooxygenase-2 selective inhibitors. J Med Chem. 2004 Dec 30;47(27):6749-59. | |||||
| REF 85 | The cyclooxygenase inhibitor flurbiprofen reduces radiation-induced angiogenic growth factor secretion of squamous cell carcinoma cell lines. Ann N Y Acad Sci. 2004 Dec;1030:37-42. | |||||
| REF 86 | Flurbiprofen, a cyclooxygenase inhibitor, protects mice from hepatic ischemia/reperfusion injury by inhibiting GSK-3 signaling and mitochondrial permeability transition.Mol Med.2012 Sep 25;18:1128-35. | |||||
| REF 87 | Flurbiprofen: A Nonselective Cyclooxygenase (COX) Inhibitor for Treatment of Noninfectious, Non-necrotizing Anterior Scleritis.Ocul Immunol Inflamm.2016;24(1):35-42. | |||||
| REF 88 | Structure-based design, synthesis, and biological evaluation of indomethacin derivatives as cyclooxygenase-2 inhibiting nitric oxide donors. J Med Chem. 2007 Dec 13;50(25):6367-82. | |||||
| REF 89 | Pharmacokinetic and pharmacodynamic aspects of the ideal COX-2 inhibitor: a pharmacologist's perspective. Clin Exp Rheumatol. 2001 Nov-Dec;19(6 Suppl 25):S51-7. | |||||
| REF 90 | Designed multiple ligands. An emerging drug discovery paradigm. J Med Chem. 2005 Oct 20;48(21):6523-43. | |||||
| REF 91 | Diazen-1-ium-1,2-diolated nitric oxide donor ester prodrugs of 5-(4-hydroxymethylphenyl)-1-(4-aminosulfonylphenyl)-3-trifluoromethyl-1H-pyrazole an... Bioorg Med Chem. 2008 Nov 15;16(22):9694-8. | |||||
| REF 92 | Selective synthesis and biological evaluation of sulfate-conjugated resveratrol metabolites. J Med Chem. 2010 Jul 8;53(13):5033-43. | |||||
| REF 93 | Synthesis and antiinflammatory activity of coumarin derivatives. J Med Chem. 2005 Oct 6;48(20):6400-8. | |||||
| REF 94 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 1375). | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

