Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T73476
(Former ID: TTDS00467)
|
|||||
| Target Name |
Antithrombin-III (ATIII)
|
|||||
| Synonyms |
SERPINC1; ATIII; AT3
Click to Show/Hide
|
|||||
| Gene Name |
SERPINC1
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 4 Target-related Diseases | + | ||||
| 1 | Coagulation defect [ICD-11: 3B10] | |||||
| 2 | Coronary thrombosis [ICD-11: BA43] | |||||
| 3 | Deep vein thrombosis [ICD-11: BD71] | |||||
| 4 | Tinnitus [ICD-11: MC41] | |||||
| Function |
Most important serine protease inhibitorin plasma that regulates the blood coagulation cascade. At-iii inhibits thrombin as well as factors ixa, xa and xia. Its inhibitory activity is greatly enhanced in the presence of heparin.
Click to Show/Hide
|
|||||
| BioChemical Class |
Serpin protein
|
|||||
| UniProt ID | ||||||
| Sequence |
MYSNVIGTVTSGKRKVYLLSLLLIGFWDCVTCHGSPVDICTAKPRDIPMNPMCIYRSPEK
KATEDEGSEQKIPEATNRRVWELSKANSRFATTFYQHLADSKNDNDNIFLSPLSISTAFA MTKLGACNDTLQQLMEVFKFDTISEKTSDQIHFFFAKLNCRLYRKANKSSKLVSANRLFG DKSLTFNETYQDISELVYGAKLQPLDFKENAEQSRAAINKWVSNKTEGRITDVIPSEAIN ELTVLVLVNTIYFKGLWKSKFSPENTRKELFYKADGESCSASMMYQEGKFRYRRVAEGTQ VLELPFKGDDITMVLILPKPEKSLAKVEKELTPEVLQEWLDELEEMMLVVHMPRFRIEDG FSLKEQLQDMGLVDLFSPEKSKLPGIVAEGRDDLYVSDAFHKAFLEVNEEGSEAAASTAV VIAGRSLNPNRVTFKANRPFLVFIREVPLNTIIFMGRVANPCVK Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | PDB | ||||
| HIT2.0 ID | T26SKO | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 3 Approved Drugs | + | ||||
| 1 | Ardeparin | Drug Info | Approved | Venous thrombosis | [3], [4] | |
| 2 | Heparin Calcium | Drug Info | Approved | Coagulation defect | [5] | |
| 3 | Sulodexide | Drug Info | Approved | Tinnitus | [6] | |
| Clinical Trial Drug(s) | [+] 9 Clinical Trial Drugs | + | ||||
| 1 | Heparin low molecular weight | Drug Info | Phase 3 | Thrombosis | [7] | |
| 2 | KW-3357 | Drug Info | Phase 3 | Coagulation defect | [8] | |
| 3 | Unfractionated heparin | Drug Info | Phase 3 | Thrombosis | [9] | |
| 4 | M118 | Drug Info | Phase 2 | Acute coronary syndrome | [10] | |
| 5 | O-desulfated heparin | Drug Info | Phase 2 | Chronic obstructive pulmonary disease | [11] | |
| 6 | PMX-60056 | Drug Info | Phase 2 | Coagulation defect | [12] | |
| 7 | MER-102 | Drug Info | Phase 1 | Thrombosis | [13] | |
| 8 | OPK-0018 | Drug Info | Phase 1 | Asthma | [14] | |
| 9 | RO-14 | Drug Info | Phase 1 | Phlebothrombosis | [15] | |
| Discontinued Drug(s) | [+] 1 Discontinued Drugs | + | ||||
| 1 | Deligoparin sodium | Drug Info | Discontinued in Phase 3 | Ulcerative colitis | [16] | |
| Mode of Action | [+] 4 Modes of Action | + | ||||
| Modulator | [+] 10 Modulator drugs | + | ||||
| 1 | Ardeparin | Drug Info | [17] | |||
| 2 | Heparin Calcium | Drug Info | [17] | |||
| 3 | Heparin low molecular weight | Drug Info | [20] | |||
| 4 | KW-3357 | Drug Info | [21] | |||
| 5 | Unfractionated heparin | Drug Info | [22] | |||
| 6 | O-desulfated heparin | Drug Info | [24] | |||
| 7 | RO-14 | Drug Info | [28] | |||
| 8 | Deligoparin sodium | Drug Info | [27] | |||
| 9 | LHD-4 | Drug Info | [27] | |||
| 10 | Org-36764 | Drug Info | [30] | |||
| Activator | [+] 1 Activator drugs | + | ||||
| 1 | Sulodexide | Drug Info | [18], [19] | |||
| Inhibitor | [+] 5 Inhibitor drugs | + | ||||
| 1 | M118 | Drug Info | [23] | |||
| 2 | MER-102 | Drug Info | [26] | |||
| 3 | Alpha-D-Mannose | Drug Info | [29] | |||
| 4 | Heparin Pentasaccharide | Drug Info | [29] | |||
| 5 | N-Formylmethionine | Drug Info | [29] | |||
| Antagonist | [+] 2 Antagonist drugs | + | ||||
| 1 | PMX-60056 | Drug Info | [25] | |||
| 2 | OPK-0018 | Drug Info | [27] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: 2-(Acetylamino)-2-Deoxy-a-D-Glucopyranose | Ligand Info | |||||
| Structure Description | Crystal structure at 2.6A of the ternary complex between antithrombin, a P14-P8 reactive loop peptide, and an exogenous tetrapeptide | PDB:1JVQ | ||||
| Method | X-ray diffraction | Resolution | 2.60 Å | Mutation | No | [31] |
| PDB Sequence |
VDICTAKPRD
14 IPMNPMCIYN45 RRVWELSKAN55 SRFATTFYQH65 LADSKNDNDN75 IFLSPLSIST 85 AFAMTKLGAC95 NDTLQQLMEV105 FKFDTISEKT115 SDQIHFFFAK125 LNCRLYRKAN 135 KSSKLVSANR145 LFGDKSLTFN155 ETYQDISELV165 YGAKLQPLDF175 KENAEQSRAA 185 INKWVSNKTE195 GRITDVIPSE205 AINELTVLVL215 VNTIYFKGLW225 KSKFSPENTR 235 KELFYKADGE245 SCSASMMYQE255 GKFRYRRVAE265 GTQVLELPFK275 GDDITMVLIL 285 PKPEKSLAKV295 EKELTPEVLQ305 EWLDELEEMM315 LVVHMPRFRI325 EDGFSLKEQL 335 QDMGLVDLFS345 PEKSKLPGIV355 AEGRDDLYVS365 DAFHKAFLEV375 NEEGSEAAAS 385 TAVVIAGRSL395 NPNRVTFKAN405 RPFLVFIREV415 PLNTIIFMGR425 VANPCV |
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target and Ligand Pair | ||||||
| Ligand Name: 2-acetamido-2-deoxy-beta-D-allopyranose | Ligand Info | |||||
| Structure Description | THE 2.6 A STRUCTURE OF ANTITHROMBIN INDICATES A CONFORMATIONAL CHANGE AT THE HEPARIN BINDING SITE | PDB:2ANT | ||||
| Method | X-ray diffraction | Resolution | 2.60 Å | Mutation | No | [32] |
| PDB Sequence |
ICTAKDIPMN
18 PMCIYRSATN45 RRVWELSKAN55 SRFATTFYQH65 LADSKNDNDN75 IFLSPLSIST 85 AFAMTKLGAC95 NDTLQQLMEV105 FKFDTISEKT115 SDQIHFFFAK125 LNCRLYRKAN 135 KSSKLVSANR145 LFGDKSLTFN155 ETYQDISELV165 YGAKLQPLDF175 KENAEQSRAA 185 INKWVSNKTE195 GRITDVIPSE205 AINELTVLVL215 VNTIYFKGLW225 KSKFSPENTR 235 KELFYKADGE245 SCSASMMYQE255 GKFRYRRVAE265 GTQVLELPFK275 GDDITMVLIL 285 PKPEKSLAKV295 EKELTPEVLQ305 EWLDELEEMM315 LVVHMPRFRI325 EDGFSLKEQL 335 QDMGLVDLFS345 PEKSKLPGIV355 AEGRDDLYVS365 DAFHKAFLEV375 NEEGSEAAAS 385 TAVVIAGRSL395 NRPFLVFIRE414 VPLNTIIFMG424 RVANPCVK
|
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Complement and coagulation cascades | hsa04610 | Affiliated Target |
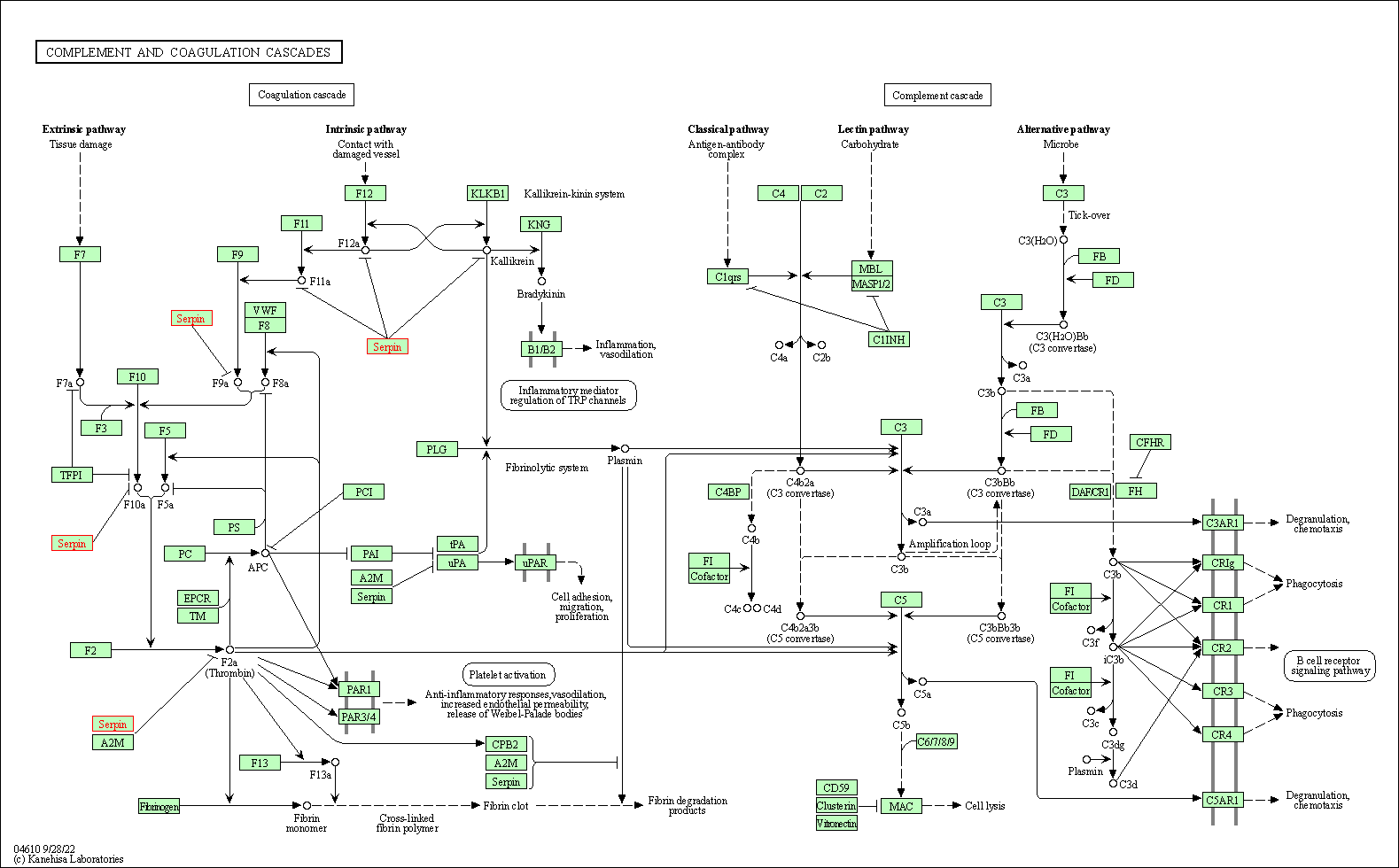
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Degree | 17 | Degree centrality | 1.83E-03 | Betweenness centrality | 4.17E-04 |
|---|---|---|---|---|---|
| Closeness centrality | 1.96E-01 | Radiality | 1.34E+01 | Clustering coefficient | 2.43E-01 |
| Neighborhood connectivity | 1.15E+01 | Topological coefficient | 1.21E-01 | Eccentricity | 13 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) | ||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 1 KEGG Pathways | + | ||||
| 1 | Complement and coagulation cascades | |||||
| Panther Pathway | [+] 1 Panther Pathways | + | ||||
| 1 | Blood coagulation | |||||
| PID Pathway | [+] 1 PID Pathways | + | ||||
| 1 | Glypican 1 network | |||||
| Reactome | [+] 2 Reactome Pathways | + | ||||
| 1 | Intrinsic Pathway of Fibrin Clot Formation | |||||
| 2 | Common Pathway of Fibrin Clot Formation | |||||
| WikiPathways | [+] 2 WikiPathways | + | ||||
| 1 | Complement and Coagulation Cascades | |||||
| 2 | Formation of Fibrin Clot (Clotting Cascade) | |||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Oral heparin: status review. Thromb J. 2006; 4: 6. | |||||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4214). | |||||
| REF 3 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6811). | |||||
| REF 4 | Pharmacologic profile of certoparin. Expert Opin Investig Drugs. 1999 Mar;8(3):315-27. | |||||
| REF 5 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||||
| REF 6 | Emerging drugs for diabetic foot ulcers. Expert Opin Emerg Drugs. 2006 Nov;11(4):709-24. | |||||
| REF 7 | Low-molecular-weight heparin in patients with advanced cancer: a phase 3 clinical trial. Mayo Clin Proc. 2006 Jun;81(6):758-67. | |||||
| REF 8 | ClinicalTrials.gov (NCT01384903) An Open-label Study of KW-3357. U.S. National Institutes of Health. | |||||
| REF 9 | Bivalirudin as a replacement for unfractionated heparin in unstable angina/non-ST-elevation myocardial infarction: observations from the TIMI 8 trial. The Thrombolysis in Myocardial Infarction. Am Heart J. 2002 Feb;143(2):229-34. | |||||
| REF 10 | Clinical pipeline report, company report or official report of Momenta Pharmaceuticals. | |||||
| REF 11 | ClinicalTrials.gov (NCT01461915) Efficacy & Safety of ODSH (2-0, 3-0 Desulfated Heparin) in Patients With Metastatic Pancreatic Cancer Treated With Gemcitabine & Abraxane. U.S. National Institutes ofHealth. | |||||
| REF 12 | ClinicalTrials.gov (NCT01312935) Reversal of Heparin in Patients Undergoing Percutaneous Coronary Intervention (PCI). U.S. National Institutes of Health. | |||||
| REF 13 | Fondaparinux sodium. Drugs Today (Barc). 2002 Mar;38(3):185-94. | |||||
| REF 14 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800034071) | |||||
| REF 15 | ClinicalTrials.gov (NCT00629733) Clinical Trial to Evaluate the Tolerability and Pharmacokinetics of a New Ultra Low Molecular Weight Heparin (RO-14) Administered Subcutaneously Increasing Single-doses to Healthy Male Volunteers. U.S. National Institutes of Health. | |||||
| REF 16 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800006120) | |||||
| REF 17 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||||
| REF 18 | Review of pharmacodynamics, pharmacokinetics, and therapeutic properties of sulodexide. Med Res Rev. 1998 Jan;18(1):1-20. | |||||
| REF 19 | Management of tinnitus: oral treatment with melatonin and sulodexide. J Biol Regul Homeost Agents. 2009 Apr-Jun;23(2):103-10. | |||||
| REF 20 | Effects of low molecular weight heparin on a severely antithrombin III-decreased disseminated intravascular coagulation model in rabbits. Thromb Res. 1995 Dec 1;80(5):391-8. | |||||
| REF 21 | Company report (kyowa-kirin) | |||||
| REF 22 | Heparin with low affinity to antithrombin III inhibits the activation of prothrombin in normal plasma. Thromb Res. 1982 Nov 15;28(4):487-97. | |||||
| REF 23 | Clinical pipeline report, company report or official report of Momentapharma. | |||||
| REF 24 | Structural determinants of the capacity of heparin to inhibit the formation of the human amplification C3 convertase. J Clin Invest. 1981 Jan;67(1):223-8. | |||||
| REF 25 | Parenteral Anticoagulants: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Correction in: Chest. 2013 August; 144(2): 721. | |||||
| REF 26 | Clinical pipeline report, company report or official report of Merrion's GIPET technology. | |||||
| REF 27 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 2632). | |||||
| REF 28 | Pharmacological effects and clinical applications of ultra low molecular weight heparins. Drug Discov Ther. 2014 Feb;8(1):1-10. | |||||
| REF 29 | How many drug targets are there Nat Rev Drug Discov. 2006 Dec;5(12):993-6. | |||||
| REF 30 | Pre-clinical pharmacological profile of the novel glycoconjugate Org 36764 with both factor Xa and thrombin (IIa) inhibitory activities. Thromb Haemost. 2000 Oct;84(4):611-20. | |||||
| REF 31 | How small peptides block and reverse serpin polymerisation. J Mol Biol. 2004 Sep 17;342(3):931-41. | |||||
| REF 32 | The 2.6 A structure of antithrombin indicates a conformational change at the heparin binding site. J Mol Biol. 1997 Feb 28;266(3):601-9. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

