Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T77009
(Former ID: TTDI00956)
|
|||||
| Target Name |
Thioredoxin reductase (PRDX5)
|
|||||
| Synonyms |
Thioredoxin peroxidase PMP20; TPx type VI; SBBI10; PrxV; Prx-V; Peroxisomal antioxidant enzyme; Peroxiredoxin5, mitochondrial; Peroxiredoxin-5, mitochondrial; Peroxiredoxin V; PLP; Liver tissue 2Dpage spot 71B; Liver tissue 2D-page spot 71B; Antioxidant enzyme B166; Alu corepressor 1; AOEB166; ACR1
Click to Show/Hide
|
|||||
| Gene Name |
PRDX5
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 1 Target-related Diseases | + | ||||
| 1 | Brain cancer [ICD-11: 2A00] | |||||
| Function |
Plays a role in cell protection against oxidative stress by detoxifying peroxides and as sensor of hydrogen peroxide-mediated signaling events. Thiol-specific peroxidase that catalyzes the reduction of hydrogen peroxide and organic hydroperoxides to water and alcohols, respectively.
Click to Show/Hide
|
|||||
| BioChemical Class |
Peroxide acceptor oxidoreductase
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 1.11.1.15
|
|||||
| Sequence |
MGLAGVCALRRSAGYILVGGAGGQSAAAAARRYSEGEWASGGVRSFSRAAAAMAPIKVGD
AIPAVEVFEGEPGNKVNLAELFKGKKGVLFGVPGAFTPGCSKTHLPGFVEQAEALKAKGV QVVACLSVNDAFVTGEWGRAHKAEGKVRLLADPTGAFGKETDLLLDDSLVSIFGNRRLKR FSMVVQDGIVKALNVEPDGTGLTCSLAPNIISQL Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| ADReCS ID | BADD_A05676 | |||||
| HIT2.0 ID | T41B8C | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 1 Approved Drugs | + | ||||
| 1 | Motexafin gadolinium | Drug Info | Approved | Brain cancer | [2] | |
| Mode of Action | [+] 1 Modes of Action | + | ||||
| Modulator | [+] 1 Modulator drugs | + | ||||
| 1 | Motexafin gadolinium | Drug Info | [1] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Catechol | Ligand Info | |||||
| Structure Description | HUMAN PEROXIREDOXIN 5 with a fragment | PDB:4K7I | ||||
| Method | X-ray diffraction | Resolution | 2.25 Å | Mutation | No | [5] |
| PDB Sequence |
SAPIKVGDAI
9 PAVEVFEGEP19 GNKVNLAELF29 KGKKGVLFGV39 PGAFTPGCSK49 THLPGFVEQA 59 EALKAKGVQV69 VACLSVNDAF79 VTGEWGRAHK89 AEGKVRLLAD99 PTGAFGKETD 109 LLLDDSLVSI119 FGNRRLKRFS129 MVVQDGIVKA139 LNVEPDGTGL149 TCSLAPNIIS 159 QL
|
|||||
|
|
||||||
| Ligand Name: (4S,5S)-1,2-Dithiane-4,5-diol | Ligand Info | |||||
| Structure Description | wild type human PrxV with DTT bound as a competitive inhibitor | PDB:3MNG | ||||
| Method | X-ray diffraction | Resolution | 1.45 Å | Mutation | No | [6] |
| PDB Sequence |
APIKVGDAIP
10 AVEVFEGEPG20 NKVNLAELFK30 GKKGVLFGVP40 GAFTPGCSKT50 HLPGFVEQAE 60 ALKAKGVQVV70 ACLSVNDAFV80 TGEWGRAHKA90 EGKVRLLADP100 TGAFGKETDL 110 LLDDSLVSIF120 GNRRLKRFSM130 VVQDGIVKAL140 NVEPDGTGLT150 CSLAPNIISQ 160 L
|
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Peroxisome | hsa04146 | Affiliated Target |
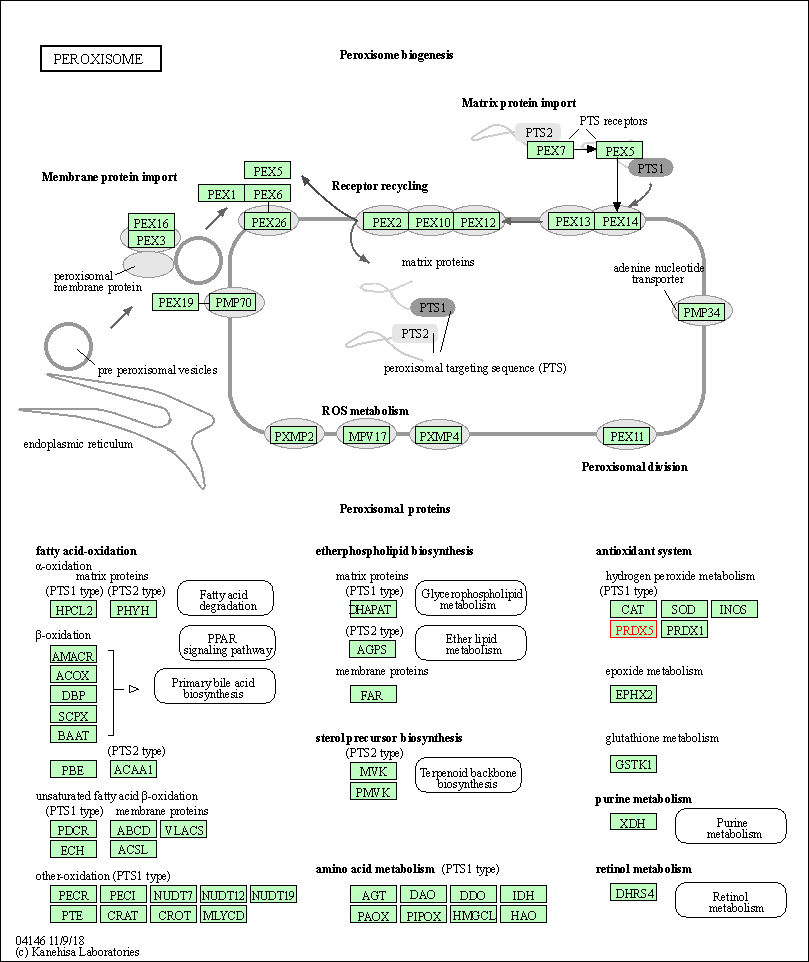
|
| Class: Cellular Processes => Transport and catabolism | Pathway Hierarchy | ||
| Degree | 5 | Degree centrality | 5.37E-04 | Betweenness centrality | 9.69E-05 |
|---|---|---|---|---|---|
| Closeness centrality | 1.96E-01 | Radiality | 1.34E+01 | Clustering coefficient | 3.00E-01 |
| Neighborhood connectivity | 7.20E+00 | Topological coefficient | 2.77E-01 | Eccentricity | 12 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-interacting Proteins | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Mechanism of inhibition of ribonucleotide reductase with motexafin gadolinium (MGd). Biochem Biophys Res Commun. 2009 Feb 13;379(3):775-9. | |||||
| REF 2 | Photodynamic therapy: applications in atherosclerotic vascular disease with motexafin lutetium. Catheter Cardiovasc Interv. 2002 Nov;57(3):387-94. | |||||
| REF 3 | ClinicalTrials.gov (NCT04419389) APR-246 in Combination With Ibrutinib or Venetoclax-R in Subjects With TP53-Mutant R/R Non Hodgkin Lymphomas (NHL) (R/R). U.S. National Institutes of Health. | |||||
| REF 4 | Thioredoxin reductase inhibitors: a patent review.Expert Opin Ther Pat. 2017 May;27(5):547-556. | |||||
| REF 5 | Comparing binding modes of analogous fragments using NMR in fragment-based drug design: application to PRDX5. PLoS One. 2014 Jul 15;9(7):e102300. | |||||
| REF 6 | Structural evidence that peroxiredoxin catalytic power is based on transition-state stabilization. J Mol Biol. 2010 Sep 10;402(1):194-209. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

