Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T83875
(Former ID: TTDS00289)
|
|||||
| Target Name |
Monoamine oxidase type A (MAO-A)
|
|||||
| Synonyms |
Monoamine oxidase A; Amine oxidase [flavin-containing] A
Click to Show/Hide
|
|||||
| Gene Name |
MAOA
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 2 Target-related Diseases | + | ||||
| 1 | Depression [ICD-11: 6A70-6A7Z] | |||||
| 2 | Parkinsonism [ICD-11: 8A00] | |||||
| Function |
MAOA preferentially oxidizes biogenic amines such as 5-hydroxytryptamine (5-HT), norepinephrine and epinephrine. Catalyzes the oxidative deamination of biogenic and xenobiotic amines and has important functions in the metabolism of neuroactive and vasoactive amines in the central nervous system and peripheral tissues.
Click to Show/Hide
|
|||||
| BioChemical Class |
CH-NH(2) donor oxidoreductase
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 1.4.3.4
|
|||||
| Sequence |
MENQEKASIAGHMFDVVVIGGGISGLSAAKLLTEYGVSVLVLEARDRVGGRTYTIRNEHV
DYVDVGGAYVGPTQNRILRLSKELGIETYKVNVSERLVQYVKGKTYPFRGAFPPVWNPIA YLDYNNLWRTIDNMGKEIPTDAPWEAQHADKWDKMTMKELIDKICWTKTARRFAYLFVNI NVTSEPHEVSALWFLWYVKQCGGTTRIFSVTNGGQERKFVGGSGQVSERIMDLLGDQVKL NHPVTHVDQSSDNIIIETLNHEHYECKYVINAIPPTLTAKIHFRPELPAERNQLIQRLPM GAVIKCMMYYKEAFWKKKDYCGCMIIEDEDAPISITLDDTKPDGSLPAIMGFILARKADR LAKLHKEIRKKKICELYAKVLGSQEALHPVHYEEKNWCEEQYSGGCYTAYFPPGIMTQYG RVIRQPVGRIFFAGTETATKWSGYMEGAVEAGERAAREVLNGLGKVTEKDIWVQEPESKD VPAVEITHTFWERNLPSVSGLLKIIGFSTSVTALGFVLYKYKLLPRS Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | PDB | ||||
| HIT2.0 ID | T21DZX | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 4 Approved Drugs | + | ||||
| 1 | Clorgyline | Drug Info | Approved | Parkinson disease | [2], [3], [4] | |
| 2 | Isocarboxazid | Drug Info | Approved | Depression | [5], [6] | |
| 3 | Moclobemide | Drug Info | Approved | Depression | [7], [8] | |
| 4 | Tranylcypromine | Drug Info | Approved | Major depressive disorder | [4] | |
| Clinical Trial Drug(s) | [+] 5 Clinical Trial Drugs | + | ||||
| 1 | CHF-3381 | Drug Info | Phase 2 | Neuropathic pain | [9], [10] | |
| 2 | CX157 | Drug Info | Phase 2 | Mood disorder | [11] | |
| 3 | Ladostigil | Drug Info | Phase 2 | Alzheimer disease | [12] | |
| 4 | PIPERINE | Drug Info | Phase 1/2 | Vitiligo | [13], [14] | |
| 5 | Desoxypeganine | Drug Info | Phase 1 | Alcohol dependence | [15] | |
| Patented Agent(s) | [+] 1 Patented Agents | + | ||||
| 1 | Schiff base compound 1 | Drug Info | Patented | Alzheimer disease | [16] | |
| Discontinued Drug(s) | [+] 8 Discontinued Drugs | + | ||||
| 1 | Befloxatone | Drug Info | Discontinued in Phase 3 | Major depressive disorder | [17], [18] | |
| 2 | Brofaromine | Drug Info | Discontinued in Phase 2 | Anxiety disorder | [20] | |
| 3 | CS-722 | Drug Info | Discontinued in Phase 2 | Epilepsy | [21] | |
| 4 | RS-8359 | Drug Info | Discontinued in Phase 2 | Major depressive disorder | [22] | |
| 5 | AS602868 | Drug Info | Discontinued in Phase 1 | Multiple myeloma | [23] | |
| 6 | BW-1370U87 | Drug Info | Discontinued in Phase 1 | Major depressive disorder | [24] | |
| 7 | Bifemelane | Drug Info | Terminated | Alzheimer disease | [25] | |
| 8 | E-2011 | Drug Info | Terminated | Anxiety disorder | [26] | |
| Mode of Action | [+] 3 Modes of Action | + | ||||
| Inhibitor | [+] 204 Inhibitor drugs | + | ||||
| 1 | Clorgyline | Drug Info | [3] | |||
| 2 | Isocarboxazid | Drug Info | [27] | |||
| 3 | Moclobemide | Drug Info | [28] | |||
| 4 | Tranylcypromine | Drug Info | [1], [29] | |||
| 5 | Psoralen | Drug Info | [30] | |||
| 6 | TRYPTAMINE | Drug Info | [31] | |||
| 7 | CHF-3381 | Drug Info | [10] | |||
| 8 | CX157 | Drug Info | [32] | |||
| 9 | Ladostigil | Drug Info | [12] | |||
| 10 | PIPERINE | Drug Info | [33] | |||
| 11 | Cyclic peptide derivative 1 | Drug Info | [34] | |||
| 12 | GypensapogeninA | Drug Info | [35] | |||
| 13 | GypensapogeninB | Drug Info | [35] | |||
| 14 | Harmine | Drug Info | [33], [36] | |||
| 15 | Heteroaryl-cyclopropylamine derivative 1 | Drug Info | [34] | |||
| 16 | HouttuynoidA | Drug Info | [35] | |||
| 17 | IncarviatoneA | Drug Info | [35] | |||
| 18 | KadcoccitoneA | Drug Info | [35] | |||
| 19 | MyriberineA | Drug Info | [35] | |||
| 20 | N-(2-phenylcyclopropyl) amino acid derivative 3 | Drug Info | [34] | |||
| 21 | PhyllanthoidA | Drug Info | [35] | |||
| 22 | PMID25399762-Compound-Figure1-Aphanamixoid A | Drug Info | [35] | |||
| 23 | PMID25399762-Compound-Figure1-Chukrasone A | Drug Info | [35] | |||
| 24 | PMID25399762-Compound-Figure1-Eryngiolide A | Drug Info | [35] | |||
| 25 | PMID25399762-Compound-Figure1-Sarcaboside A | Drug Info | [35] | |||
| 26 | PMID25399762-Compound-Figure1-Sarcaboside B | Drug Info | [35] | |||
| 27 | PMID25399762-Compound-Figure2-Artoxanthochromane | Drug Info | [35] | |||
| 28 | PMID25399762-Compound-Figure2-Spirooliganone B | Drug Info | [35] | |||
| 29 | PMID25399762-Compound-Figure3-Aspeverin | Drug Info | [35] | |||
| 30 | PMID25399762-Compound-Figure3-Fluevirosine A | Drug Info | [35] | |||
| 31 | PMID25399762-Compound-Figure3-Lycojaponicumin A | Drug Info | [35] | |||
| 32 | PMID25399762-Compound-Figure3-Lycojaponicumin B | Drug Info | [35] | |||
| 33 | PMID25399762-Compound-Figure3-Lycojaponicumin C | Drug Info | [35] | |||
| 34 | PMID25399762-Compound-Table 5-8 | Drug Info | [35] | |||
| 35 | PMID25399762-Compound-Table 5-O-methyl-M30 | Drug Info | [35] | |||
| 36 | PMID29324067-Compound-25 | Drug Info | [16] | |||
| 37 | PMID29757691-Compound-4 | Drug Info | [37] | |||
| 38 | Secondary and tertiary (hetero)arylamide derivative 2 | Drug Info | [16] | |||
| 39 | Tarnylcypromine derivative 2 | Drug Info | [34] | |||
| 40 | Tarnylcypromine derivative 3 | Drug Info | [34] | |||
| 41 | Tetra-hydro-isoquinoline derivative 1 | Drug Info | [37] | |||
| 42 | Tetra-hydro-isoquinoline derivative 2 | Drug Info | [37] | |||
| 43 | Tetra-hydro-isoquinoline derivative 3 | Drug Info | [37] | |||
| 44 | Tetra-hydro-isoquinoline derivative 4 | Drug Info | [37] | |||
| 45 | Befloxatone | Drug Info | [38] | |||
| 46 | CS-722 | Drug Info | [40] | |||
| 47 | RS-8359 | Drug Info | [41] | |||
| 48 | AS602868 | Drug Info | [30] | |||
| 49 | BW-1370U87 | Drug Info | [4], [42] | |||
| 50 | (+/-)-2-(4'-Benzyloxyphenyl)thiomorpholine | Drug Info | [44] | |||
| 51 | (+/-)-2-(4'-Butoxyphenyl)thiomorpholin-5-one | Drug Info | [44] | |||
| 52 | (+/-)-2-(4'-Butoxyphenyl)thiomorpholine | Drug Info | [44] | |||
| 53 | (+/-)-2-(4'-Ethoxyphenyl)thiomorpholin-5-one | Drug Info | [44] | |||
| 54 | (+/-)-2-(4'-Ethoxyphenyl)thiomorpholine | Drug Info | [44] | |||
| 55 | (+/-)-2-(4'-Methoxyphenyl)thiomorpholin-5-one | Drug Info | [44] | |||
| 56 | (+/-)-2-(4'-Methoxyphenyl)thiomorpholine | Drug Info | [44] | |||
| 57 | (+/-)-2-(4'-Propoxyphenyl)thiomorpholine | Drug Info | [44] | |||
| 58 | (+/-)-2-Phenylthiomorpholin-5-one | Drug Info | [44] | |||
| 59 | (+/-)-2-Phenylthiomorpholine | Drug Info | [44] | |||
| 60 | (6-Benzyloxy-2-naphthyl)-2-aminopropane | Drug Info | [45] | |||
| 61 | (6-Ethoxy-2-naphthyl)-2-aminopropane | Drug Info | [45] | |||
| 62 | (6-Methoxy-2-naphthyl)-2-aminopropane | Drug Info | [45] | |||
| 63 | (6-methylthio-2-naphthyl)isopropylamine | Drug Info | [45] | |||
| 64 | (6-Propoxy-2-naphthyl)-2-aminopropane | Drug Info | [45] | |||
| 65 | (7-Benzyloxy-2-oxo-2H-chromen-4-yl)acetonitrile | Drug Info | [46] | |||
| 66 | (E)-5-(3-Chlorostyryl)isatin | Drug Info | [47] | |||
| 67 | (E)-5-Styrylisatin | Drug Info | [47] | |||
| 68 | (R)-Indan-1-yl-methyl-prop-2-ynyl-amine | Drug Info | [48] | |||
| 69 | (R,S)-N-(R-phenylethyl)-1H-pyrrole-2-carboxamide | Drug Info | [49] | |||
| 70 | (R/R)BEFLOXATONE | Drug Info | [50] | |||
| 71 | (S)-2-amino-1-(4-ethylthiophenyl)-propane | Drug Info | [51] | |||
| 72 | (S)-2-amino-1-(4-methylthiophenyl)-propane | Drug Info | [51] | |||
| 73 | (S)-2-amino-1-(4-propylthiophenyl)-propane | Drug Info | [51] | |||
| 74 | 1,2,3,4-Tetrahydro-pyrazino[1,2-a]indole | Drug Info | [52] | |||
| 75 | 1-(1-Naphthyl)-2-aminopropane | Drug Info | [45] | |||
| 76 | 1-(2-Naphthyl)-2-aminopropane | Drug Info | [45] | |||
| 77 | 1-(3-(4-chlorobenzyl)quinoxalin-2-yl)hydrazine | Drug Info | [53] | |||
| 78 | 1-(3-benzylquinoxalin-2-yl)hydrazine | Drug Info | [53] | |||
| 79 | 1-(4-(benzyloxy)phenyl)propan-2-amine | Drug Info | [45] | |||
| 80 | 1-(4-butoxyphenyl)propan-2-amine | Drug Info | [45] | |||
| 81 | 1-(4-propoxyphenyl)propan-2-amine | Drug Info | [45] | |||
| 82 | 2,3,4,5-Tetrahydro-1H-pyrido[4,3-b]indole | Drug Info | [31] | |||
| 83 | 2-(2-cycloheptylidenehydrazinyl)-4-phenylthiazole | Drug Info | [54] | |||
| 84 | 2-(2-cyclopentylidenehydrazinyl)-4-phenylthiazole | Drug Info | [54] | |||
| 85 | 2-(3,4-dimethoxyphenyl)-4,5-dihydro-1H-imidazole | Drug Info | [55] | |||
| 86 | 2-(3-benzylquinoxalin-2-ylamino)ethanol | Drug Info | [53] | |||
| 87 | 2-(4,5-dihydro-1H-imidazol-2-yl)quinoline | Drug Info | [55] | |||
| 88 | 2-(4-methoxyphenyl)-4,5-dihydro-1H-imidazole | Drug Info | [55] | |||
| 89 | 2-(5-phenyl-furan-2-yl)-4,5-dihydro-1H-imidazole | Drug Info | [56] | |||
| 90 | 2-(naphthalen-2-yl)-4,5-dihydro-1H-imidazole | Drug Info | [55] | |||
| 91 | 2-Amino-1-(4-methylthiophenyl)propane | Drug Info | [45] | |||
| 92 | 2-BFi | Drug Info | [57] | |||
| 93 | 2-Bromo-N-(2-morpholinoethyl)nicotinamide | Drug Info | [58] | |||
| 94 | 2-Chloro-N-(2-morpholinoethyl)nicotinamide | Drug Info | [58] | |||
| 95 | 2-Chloro-N-(3-morpholinopropyl)nicotinamide | Drug Info | [58] | |||
| 96 | 2-Furan-2-yl-4,5-dihydro-1H-imidazole | Drug Info | [59] | |||
| 97 | 2-oxo-N-m-tolyl-2H-chromene-3-carboxamide | Drug Info | [60] | |||
| 98 | 2-oxo-N-p-tolyl-2H-chromene-3-carboxamide | Drug Info | [60] | |||
| 99 | 2-oxo-N-phenyl-2H-chromene-3-carboxamide | Drug Info | [60] | |||
| 100 | 2-Phenoxymethyl-4,5-dihydro-1H-imidazole | Drug Info | [59] | |||
| 101 | 2-phenyl-9H-indeno[2,1-d]pyrimidine | Drug Info | [61] | |||
| 102 | 2-[7-(Benzyloxy)-2-oxo-2H-chromen-4-yl]acetamide | Drug Info | [46] | |||
| 103 | 3,4-Benzo-7-(beta-bromoallyloxy)-8-methylcoumarin | Drug Info | [62] | |||
| 104 | 3,4-Benzo-7-acetonyloxy-8-methoxycoumarin | Drug Info | [62] | |||
| 105 | 3,4-Dichloro-N-(2-methyl-1H-indol-5-yl)benzamide | Drug Info | [63] | |||
| 106 | 3-aminoacetamido-4'-methylfuro[3,2-g]coumarin | Drug Info | [64] | |||
| 107 | 3-benzyl-N-(2-morpholinoethyl)quinoxalin-2-amine | Drug Info | [53] | |||
| 108 | 3-Chloro-N-(2-methyl-1H-indol-5-yl)benzamide | Drug Info | [63] | |||
| 109 | 3-methyl-2(1H)-thioxo-4(3H)-quinazolinone | Drug Info | [65] | |||
| 110 | 4,8-Dimethyl-7-(2'-oxocyclohexyloxy)coumarin | Drug Info | [62] | |||
| 111 | 4,9-Dihydro-3H-beta-carboline | Drug Info | [52] | |||
| 112 | 4-(2-oxo-2H-chromene-3-carboxamido)benzoic acid | Drug Info | [60] | |||
| 113 | 4-(Aminomethyl)-7-(benzyloxy)-2H-chromen-2-one | Drug Info | [46] | |||
| 114 | 4-Chloro-N-(2-morpholinoethyl)nicotinamide | Drug Info | [58] | |||
| 115 | 4-Chloro-N-(3-morpholinopropyl)nicotinamide | Drug Info | [58] | |||
| 116 | 4-methyl-2H-benzofuro[3,2-g]chromen-2-one | Drug Info | [64] | |||
| 117 | 4-methyl-7-(2-oxocyclopentyloxy)-2H-chromen-2-one | Drug Info | [62] | |||
| 118 | 5,6-Dichloro-N-(2-morpholinoethyl)nicotinamide | Drug Info | [58] | |||
| 119 | 5-Azidomethyl-3-pyrrol-1-yl-oxazolidin-2-one | Drug Info | [50] | |||
| 120 | 5-Bromo-2,3,4,9-tetrahydro-1H-beta-carboline | Drug Info | [31] | |||
| 121 | 5-Bromo-4,9-dihydro-3H-beta-carboline | Drug Info | [31] | |||
| 122 | 5-Hydroxymethyl-3-pyrrol-1-yl-oxazolidin-2-one | Drug Info | [50] | |||
| 123 | 5-Methoxy-2,3,4,9-tetrahydro-1H-beta-carboline | Drug Info | [31] | |||
| 124 | 5-Methoxymethyl-3-pyrrol-1-yl-oxazolidin-2-one | Drug Info | [50] | |||
| 125 | 6,11-dihydro-5H-benzo[a]carbazole | Drug Info | [61] | |||
| 126 | 6-amino-9-methoxy-7H-furo[3,2-g]chromen-7-one | Drug Info | [62] | |||
| 127 | 6-Bromo-2,3,4,9-tetrahydro-1H-beta-carboline | Drug Info | [31] | |||
| 128 | 6-Bromo-4,9-dihydro-3H-beta-carboline | Drug Info | [31] | |||
| 129 | 6-Chloro-N-(2-morpholinoethyl)nicotinamide | Drug Info | [58] | |||
| 130 | 6-Chloro-N-(3-morpholinopropyl)nicotinamide | Drug Info | [58] | |||
| 131 | 6-Fluoro-N-(2-morpholinoethyl)nicotinamide | Drug Info | [58] | |||
| 132 | 6-Hydroxy-N-(2-morpholinoethyl)nicotinamide | Drug Info | [58] | |||
| 133 | 6-Methoxy-2,3,4,9-tetrahydro-1H-beta-carboline | Drug Info | [52] | |||
| 134 | 6-Methoxy-4,9-dihydro-3H-beta-carboline | Drug Info | [52] | |||
| 135 | 7-(3-chlorobenzyloxy)-4-carboxaldehyde-coumarin | Drug Info | [66] | |||
| 136 | 7-Acetonyloxy-3,4-cyclohexene-8-methylcoumarin | Drug Info | [62] | |||
| 137 | 7-Acetonyloxy-3,4-cyclopentene-8-methylcoumarin | Drug Info | [62] | |||
| 138 | 7-acetonyloxy-3-acetylamino-8-methoxycoumarin | Drug Info | [64] | |||
| 139 | 7-Bromo-2,3,4,9-tetrahydro-1H-beta-carboline | Drug Info | [31] | |||
| 140 | 7-Methoxy-2,3,4,9-tetrahydro-1H-beta-carboline | Drug Info | [59] | |||
| 141 | 7-Methoxy-9H-beta-carboline | Drug Info | [31] | |||
| 142 | 8-(3-Bromobenzyloxy)caffeine | Drug Info | [67] | |||
| 143 | 8-(3-Chlorobenzyloxy)caffeine | Drug Info | [67] | |||
| 144 | 8-(3-Fluorobenzyloxy)caffeine | Drug Info | [67] | |||
| 145 | 8-(3-Methoxybenzyloxy)caffeine | Drug Info | [67] | |||
| 146 | 8-(3-Methylbenzyloxy)caffeine | Drug Info | [67] | |||
| 147 | 8-Benzyloxycaffeine | Drug Info | [67] | |||
| 148 | 8-Bromo-2,3,4,9-tetrahydro-1H-beta-carboline | Drug Info | [31] | |||
| 149 | 8-Bromo-4,9-dihydro-3H-beta-carboline | Drug Info | [31] | |||
| 150 | 8-Methoxy-2,3,4,9-tetrahydro-1H-beta-carboline | Drug Info | [31] | |||
| 151 | 8-[(3-Trifluoromethyl)benzyloxy]caffeine | Drug Info | [67] | |||
| 152 | 9-Methyl-2,3,4,9-tetrahydro-1H-beta-carboline | Drug Info | [31] | |||
| 153 | Beta-methoxyamphetamine | Drug Info | [45], [68] | |||
| 154 | C-(1H-Indol-3-yl)-methylamine | Drug Info | [31] | |||
| 155 | CGS-19281A | Drug Info | [52] | |||
| 156 | Cis-(+/-)-2-Fluoro-1,2-diphenylcyclopropylamine | Drug Info | [69] | |||
| 157 | DECYL(DIMETHYL)PHOSPHINE OXIDE | Drug Info | [36] | |||
| 158 | Ethyl 4-(2-oxo-2H-chromene-3-carboxamido)benzoate | Drug Info | [60] | |||
| 159 | FA-70 | Drug Info | [70] | |||
| 160 | Flavin-Adenine Dinucleotide | Drug Info | [71] | |||
| 161 | HYDRAZINECARBOXAMIDE | Drug Info | [72] | |||
| 162 | IPRONIAZIDE | Drug Info | [58] | |||
| 163 | N'-(2-phenylallyl)hydrazine hydrochloride | Drug Info | [73] | |||
| 164 | N-((1H-indol-2-yl)methyl)(phenyl)methanamine | Drug Info | [74] | |||
| 165 | N-(1-Methyl-1H-indol-2-ylmethyl)-N-phenylamine | Drug Info | [74] | |||
| 166 | N-(1H-Indol-2-ylmethyl)-N-(4-phenylbutyl)amine | Drug Info | [74] | |||
| 167 | N-(1H-Indol-2-ylmethyl)-N-methyl-N-phenylamine | Drug Info | [74] | |||
| 168 | N-(1H-Indol-2-ylmethyl)-N-phenylamine | Drug Info | [74] | |||
| 169 | N-(2-benzyl),N-(1-methylpyrrol-2-ylmethyl)amine | Drug Info | [49] | |||
| 170 | N-(2-Methyl-1H-indol-5-yl)cyclohexanecarboxamide | Drug Info | [63] | |||
| 171 | N-(2-phenylethyl),N-(pyrrol-2-ylmethyl)amine | Drug Info | [49] | |||
| 172 | N-(3-Phenylpropyl)-1H-indole-2-carboxamide | Drug Info | [74] | |||
| 173 | N-(4-Ethylphenyl)-2-oxo-2H-chromene-3-carboxamide | Drug Info | [60] | |||
| 174 | N-(4-Phenylbutyl)-1H-indole-2-carboxamide | Drug Info | [74] | |||
| 175 | N-(4-phenylbutyl)-1H-pyrrole-2-carboxamide | Drug Info | [49] | |||
| 176 | N-(benzyl),N-(pyrrol-2-ylmethyl)amine | Drug Info | [49] | |||
| 177 | N-(propargyl),N-(pyrrol-2-ylmethyl)amine | Drug Info | [49] | |||
| 178 | N-2-phenylethyl-1H-pyrrole-2-carboxamide | Drug Info | [49] | |||
| 179 | N-benzyl,N-methyl-1H-pyrrole-2-carboxamide | Drug Info | [49] | |||
| 180 | N-Benzyl-(6-butoxy-2-naphthyl)-2-aminopropane | Drug Info | [45] | |||
| 181 | N-Benzyl-(6-methoxy-2-naphthyl)-2-aminopropane | Drug Info | [45] | |||
| 182 | N-benzyl-1H-pyrrole-2-carboxamide | Drug Info | [49] | |||
| 183 | N-Benzyl-N-(1H-indol-2-ylmethyl)-N-methylamine | Drug Info | [74] | |||
| 184 | N-methyl,N-(benzyl),N-(pyrrol-2-ylmethyl)amine | Drug Info | [49] | |||
| 185 | N-methyl,N-(propargyl),N-(pyrrol-2-ylmethyl)amine | Drug Info | [49] | |||
| 186 | N-Methyl,N-phenyl-1H-indole-2-carboxamide | Drug Info | [74] | |||
| 187 | N-methyl-N-(prop-2-ynyl)-1H-pyrrole-2-carboxamide | Drug Info | [49] | |||
| 188 | N-Phenyl-1-methyl-1H-indole-2-carboxamide | Drug Info | [74] | |||
| 189 | N-Phenyl-1H-indole-2-carboxamide | Drug Info | [74] | |||
| 190 | N-phenyl-1H-pyrrole-2-carboxamide | Drug Info | [49] | |||
| 191 | N-propargyl-1H-pyrrole-2-carboxamide | Drug Info | [49] | |||
| 192 | N2-[4-(benzyloxy)benzyl]glycinamide | Drug Info | [75] | |||
| 193 | N2-{4-[(3-fluorobenzyl)oxy]benzyl}glycinamide | Drug Info | [75] | |||
| 194 | N2-{4-[(4-chlorobenzyl)oxy]benzyl}glycinamide | Drug Info | [75] | |||
| 195 | NSC-656158 | Drug Info | [76] | |||
| 196 | Phenyl 4-(4,5-dihydro-1H-imidazol-2-yl)benzoate | Drug Info | [55] | |||
| 197 | PNU-22394 | Drug Info | [31] | |||
| 198 | TRACIZOLINE | Drug Info | [59] | |||
| 199 | Trans-(+/-)-2-Fluoro-1,2-diphenylcyclopropylamine | Drug Info | [69] | |||
| 200 | Trans-2-(4-chlorophenyl)-2-fluorocyclopropanamine | Drug Info | [69] | |||
| 201 | Trans-2-fluoro-2-(4-fluorophenyl)cyclopropanamine | Drug Info | [69] | |||
| 202 | Trans-2-fluoro-2-p-tolylcyclopropanamine | Drug Info | [69] | |||
| 203 | Trans-2-fluoro-2-phenylcyclopropylamin | Drug Info | [69] | |||
| 204 | TRYPTOLINE | Drug Info | [59] | |||
| Modulator | [+] 4 Modulator drugs | + | ||||
| 1 | Desoxypeganine | Drug Info | [15] | |||
| 2 | Brofaromine | Drug Info | [39] | |||
| 3 | Bifemelane | Drug Info | [25] | |||
| 4 | E-2011 | Drug Info | [43] | |||
| Antagonist | [+] 1 Antagonist drugs | + | ||||
| 1 | MMDA | Drug Info | [71] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Clorgyline | Ligand Info | |||||
| Structure Description | Human Monoamine Oxidase A in complex with Clorgyline, Crystal Form A | PDB:2BXR | ||||
| Method | X-ray diffraction | Resolution | 3.00 Å | Mutation | No | [77] |
| PDB Sequence |
HMFDVVVIGG
21 GISGLSAAKL31 LTEYGVSVLV41 LEARDRVGGR51 TYTIRNEHVD61 YVDVGGAYVG 71 PTQNRILRLS81 KELGIETYKV91 NVSERLVQYV101 KGKTYPFRGW116 NPIAYLDYNN 126 LWRTIDNMGK136 EIPTDAPWEA146 QHADKWDKMT156 MKELIDKICW166 TKTARRFAYL 176 FVNINVTSEP186 HEVSALWFLW196 YVKQCGGTTR206 IFSVGQERKF219 VGGSGQVSER 229 IMDLLGDQVK239 LNHPVTHVDQ249 SSDNIIIETL259 NHEHYECKYV269 INAIPPTLTA 279 KIHFRPELPA289 ERNQLIQRLP299 MGAVIKCMMY309 YKEAFWKKKD319 YCGCMIIEDE 329 DAPISITLDD339 TKPDGSLPAI349 MGFILARKAD359 RLAKLHKEIR369 KKKICELYAK 379 VLGSQEALHP389 VHYEEKNWCE399 EQYSGGCYTA409 YFPPGIMTQY419 GRVIRQPVGR 429 IFFAGTETAT439 KWSGYMEGAV449 EAGERAAREV459 LNGLG
|
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target and Ligand Pair | ||||||
| Ligand Name: Decyl(dimethyl)phosphine oxide | Ligand Info | |||||
| Structure Description | Crystal Structure of Human Monoamine Oxidase A (G110A) with Harmine | PDB:2Z5Y | ||||
| Method | X-ray diffraction | Resolution | 2.17 Å | Mutation | Yes | [78] |
| PDB Sequence |
HMFDVVVIGG
21 GISGLSAAKL31 LTEYGVSVLV41 LEARDRVGGR51 TYTIRNEHVD61 YVDVGGAYVG 71 PTQNRILRLS81 KELGIETYKV91 NVSERLVQYV101 KGKTYPFRAA111 FPPVWNPIAY 121 LDYNNLWRTI131 DNMGKEIPTD141 APWEAQHADK151 WDKMTMKELI161 DKICWTKTAR 171 RFAYLFVNIN181 VTSEPHEVSA191 LWFLWYVKQC201 GGTTRIFSVT211 NGGQERKFVG 221 GSGQVSERIM231 DLLGDQVKLN241 HPVTHVDQSS251 DNIIIETLNH261 EHYECKYVIN 271 AIPPTLTAKI281 HFRPELPAER291 NQLIQRLPMG301 AVIKCMMYYK311 EAFWKKKDYC 321 GCMIIEDEDA331 PISITLDDTK341 PDGSLPAIMG351 FILARKADRL361 AKLHKEIRKK 371 KICELYAKVL381 GSQEALHPVH391 YEEKNWCEEQ401 YSGGCYTAYF411 PPGIMTQYGR 421 VIRQPVGRIF431 FAGTETATKW441 SGYMEGAVEA451 GERAAREVLN461 GLGKVTEKDI 471 WVQEPESKDV481 PAVEITHTFW491 ERNLPSVSGL501 LKIIGFSTSV511 TALGFVLYKY 521 KLL
|
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target and Ligand Pair | ||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Glycine, serine and threonine metabolism | hsa00260 | Affiliated Target |
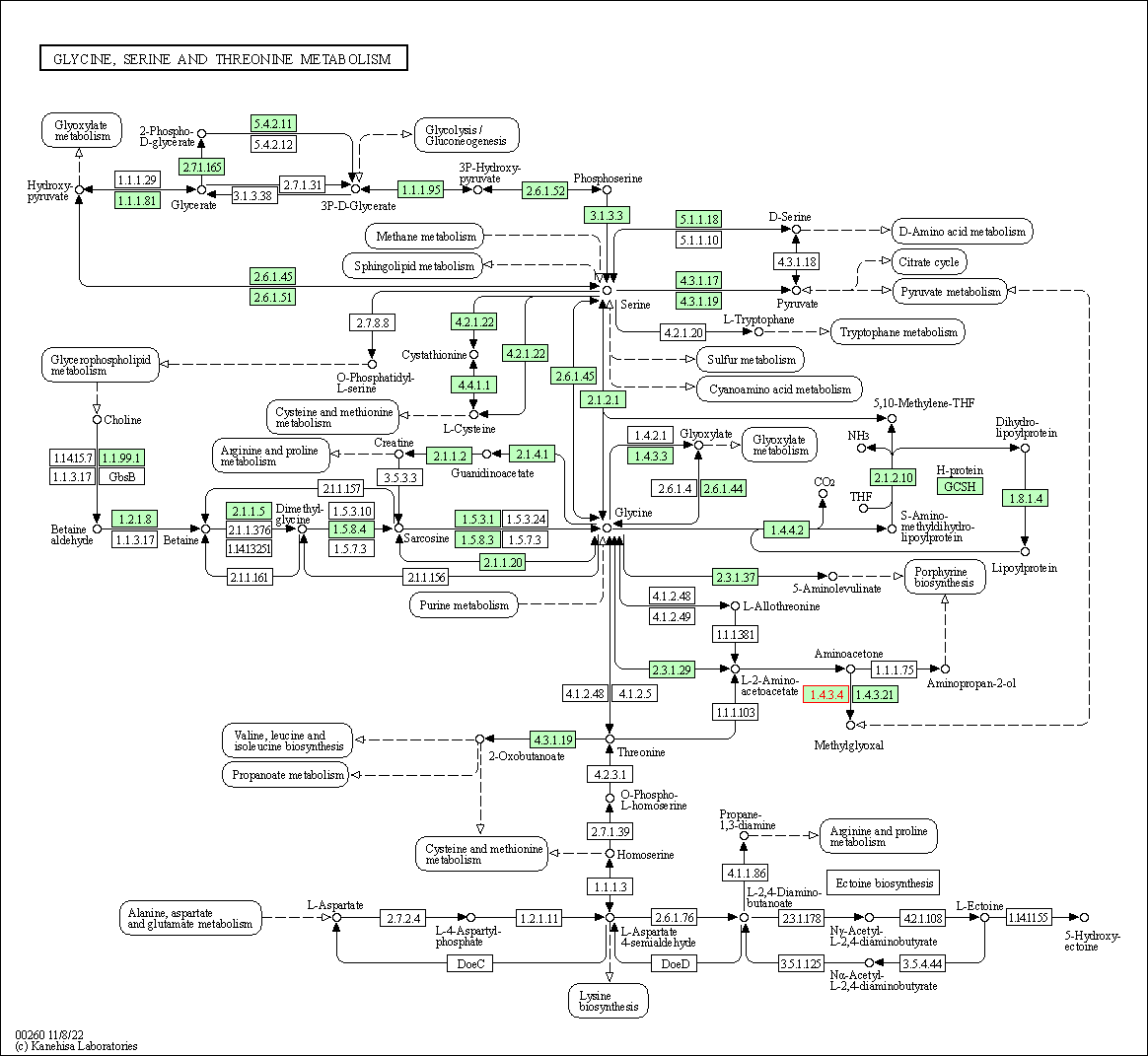
|
| Class: Metabolism => Amino acid metabolism | Pathway Hierarchy | ||
| Arginine and proline metabolism | hsa00330 | Affiliated Target |
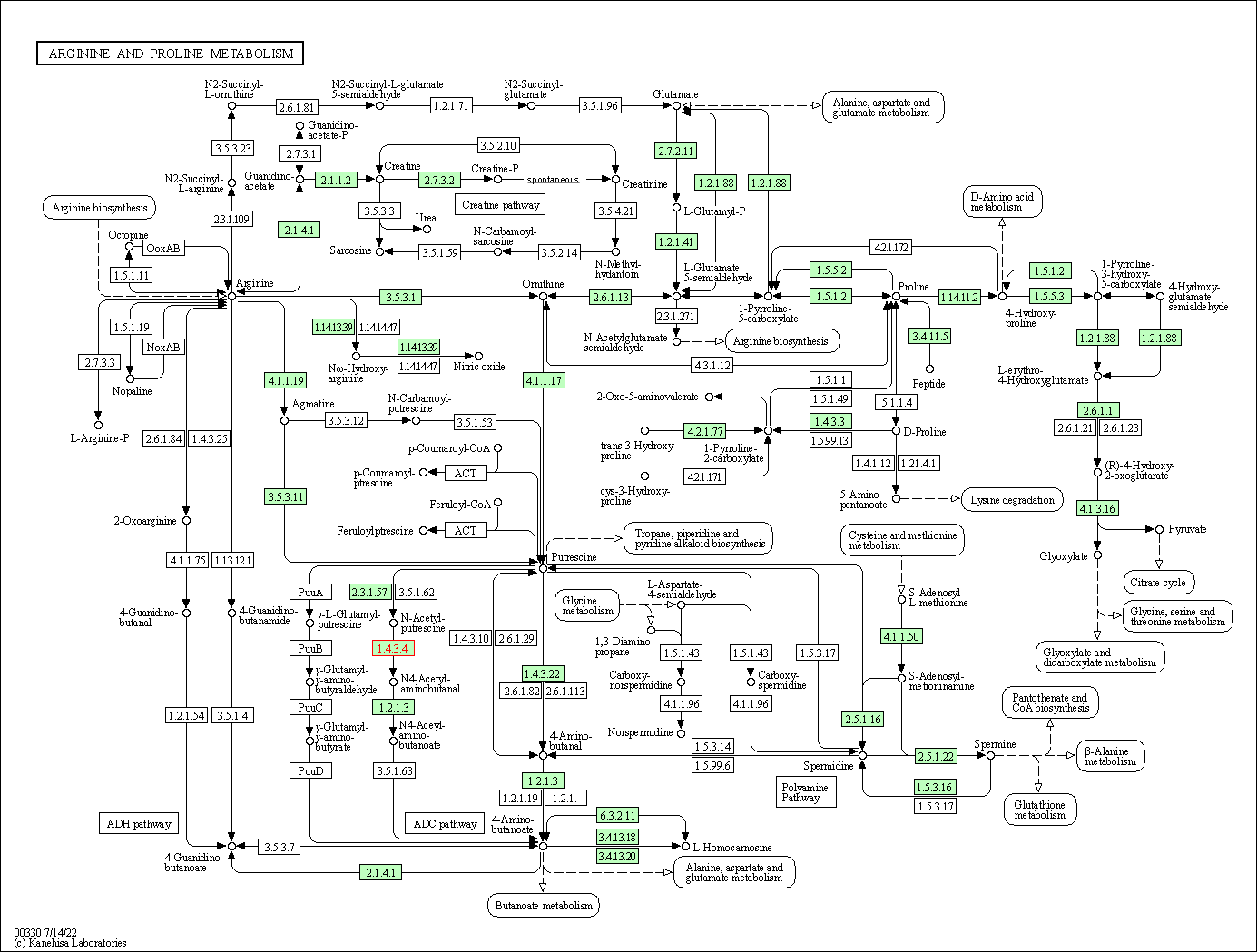
|
| Class: Metabolism => Amino acid metabolism | Pathway Hierarchy | ||
| Histidine metabolism | hsa00340 | Affiliated Target |
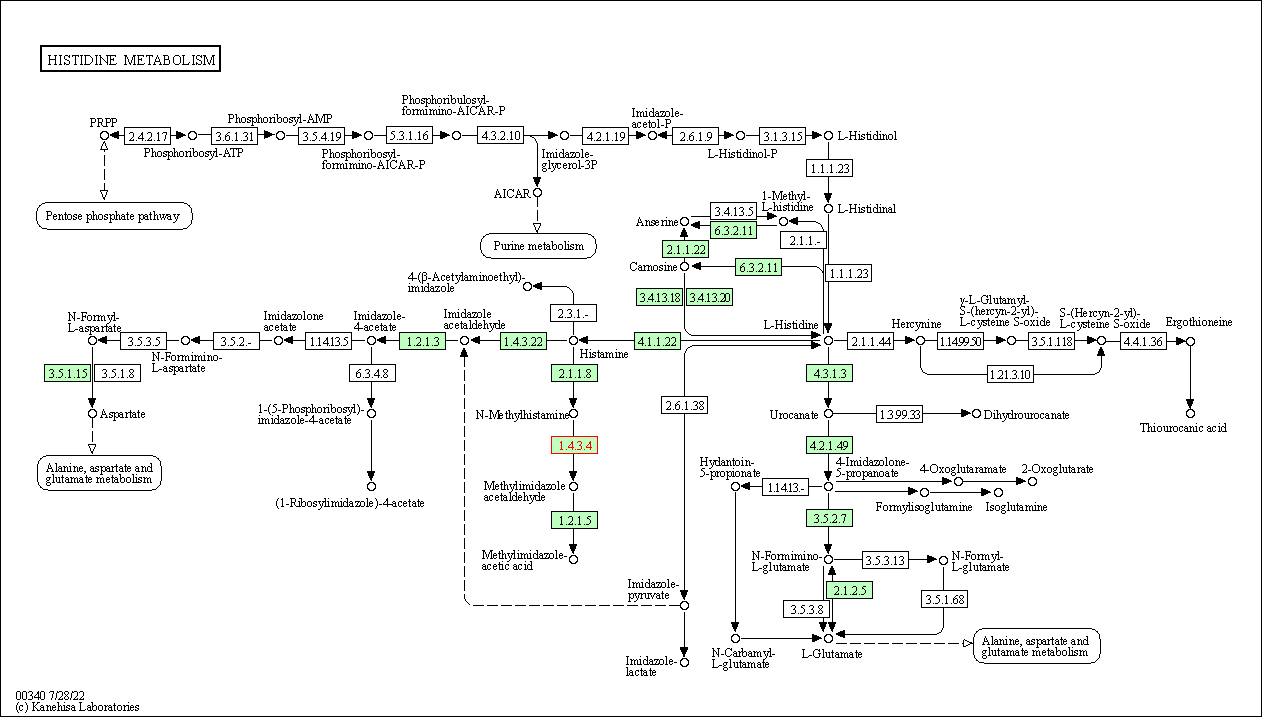
|
| Class: Metabolism => Amino acid metabolism | Pathway Hierarchy | ||
| Tyrosine metabolism | hsa00350 | Affiliated Target |

|
| Class: Metabolism => Amino acid metabolism | Pathway Hierarchy | ||
| Phenylalanine metabolism | hsa00360 | Affiliated Target |
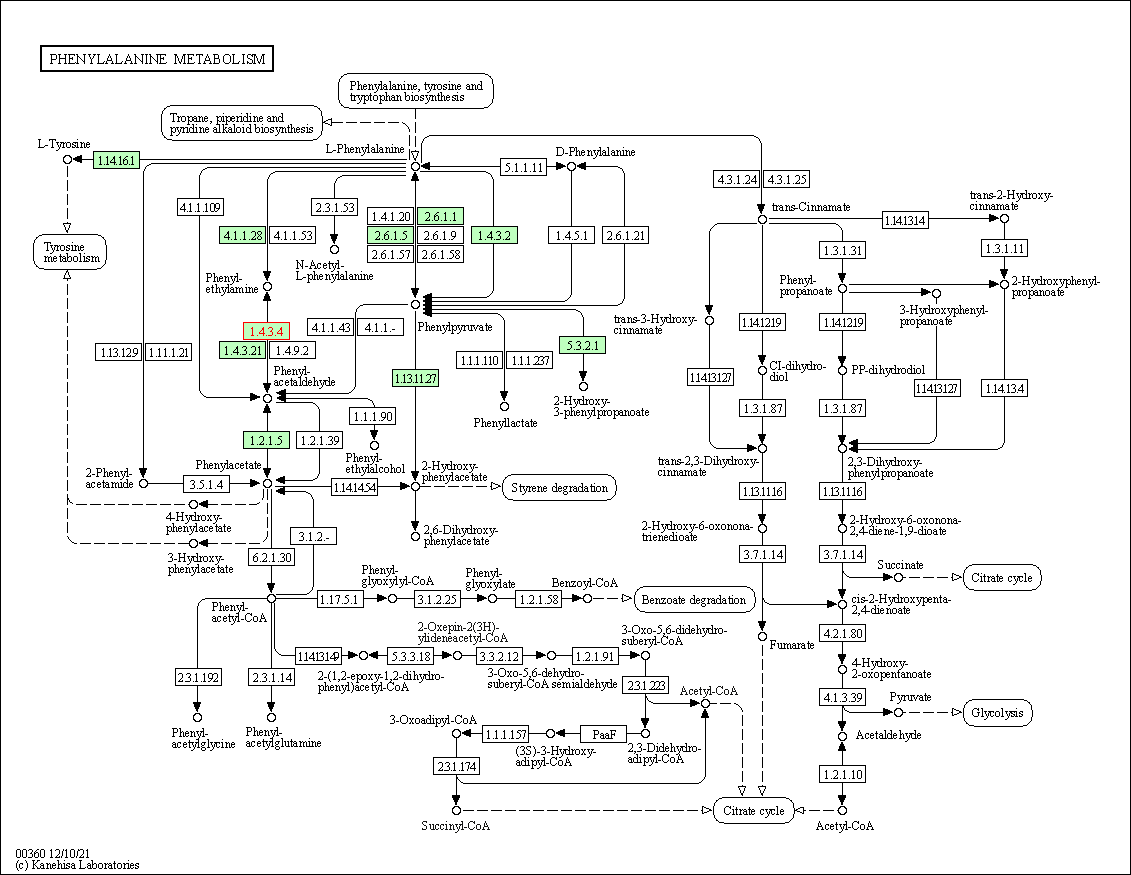
|
| Class: Metabolism => Amino acid metabolism | Pathway Hierarchy | ||
| Tryptophan metabolism | hsa00380 | Affiliated Target |
|
| Class: Metabolism => Amino acid metabolism | Pathway Hierarchy | ||
| Drug metabolism - cytochrome P450 | hsa00982 | Affiliated Target |

|
| Class: Metabolism => Xenobiotics biodegradation and metabolism | Pathway Hierarchy | ||
| Serotonergic synapse | hsa04726 | Affiliated Target |
|
| Class: Organismal Systems => Nervous system | Pathway Hierarchy | ||
| Dopaminergic synapse | hsa04728 | Affiliated Target |
|
| Class: Organismal Systems => Nervous system | Pathway Hierarchy | ||
| Click to Show/Hide the Information of Affiliated Human Pathways | |||
| Degree | 6 | Degree centrality | 6.45E-04 | Betweenness centrality | 2.87E-04 |
|---|---|---|---|---|---|
| Closeness centrality | 1.63E-01 | Radiality | 1.25E+01 | Clustering coefficient | 2.00E-01 |
| Neighborhood connectivity | 5.83E+00 | Topological coefficient | 2.22E-01 | Eccentricity | 12 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) | ||||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| Target QSAR Model | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Tramadol and another atypical opioid meperidine have exaggerated serotonin syndrome behavioural effects, but decreased analgesic effects, in genetically deficient serotonin transporter (SERT) mice. Int J Neuropsychopharmacol. 2009 Mar 11:1-11. | |||||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6636). | |||||
| REF 3 | Further investigation into the mechanism of tachykinin NK(2) receptor-triggered serotonin release from guinea-pig proximal colon. J Pharmacol Sci. 2009 May;110(1):122-6. | |||||
| REF 4 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||||
| REF 5 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7204). | |||||
| REF 6 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 011961. | |||||
| REF 7 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7428). | |||||
| REF 8 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. | |||||
| REF 9 | Indantadol, a novel NMDA antagonist and nonselective MAO inhibitor for the potential treatment of neuropathic pain. IDrugs. 2007 Sep;10(9):636-44. | |||||
| REF 10 | Emerging drugs in neuropathic pain. Expert Opin Emerg Drugs. 2007 Mar;12(1):113-26. | |||||
| REF 11 | ClinicalTrials.gov (NCT01246908) Efficacy, Safety and Tolerability of CX157 in Treatment Resistant Depression. U.S. National Institutes of Health. | |||||
| REF 12 | Ladostigil: a novel multimodal neuroprotective drug with cholinesterase and brain-selective monoamine oxidase inhibitory activities for Alzheimer's disease treatment. Curr Drug Targets. 2012 Apr;13(4):483-94. | |||||
| REF 13 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2489). | |||||
| REF 14 | ClinicalTrials.gov (NCT01383694) Effect Of Piperine In Patients With Oropharyngeal Dysphagia. U.S. National Institutes of Health. | |||||
| REF 15 | Phase I clinical trial with desoxypeganine, a new cholinesterase and selective MAO-A inhibitor: tolerance and pharmacokinetics study of escalating single oral doses. Methods Find Exp Clin Pharmacol. 2008 Mar;30(2):141-7. | |||||
| REF 16 | MAO inhibitors and their wider applications: a patent review.Expert Opin Ther Pat. 2018 Mar;28(3):211-226. | |||||
| REF 17 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6637). | |||||
| REF 18 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800001426) | |||||
| REF 19 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800005281) | |||||
| REF 20 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800000689) | |||||
| REF 21 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800003060) | |||||
| REF 22 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800000633) | |||||
| REF 23 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800009941) | |||||
| REF 24 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002739) | |||||
| REF 25 | 4-(O-benzylphenoxy)-N-methylbutylamine (bifemelane) and other 4-(O-benzylphenoxy)-N-methylalkylamines as new inhibitors of type A and B monoamine oxidase. J Neurochem. 1988 Jan;50(1):243-7. | |||||
| REF 26 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002928) | |||||
| REF 27 | MAOIs in the contemporary treatment of depression. Neuropsychopharmacology. 1995 May;12(3):185-219. | |||||
| REF 28 | Efficacy of citalopram and moclobemide in patients with social phobia: some preliminary findings. Hum Psychopharmacol. 2002 Dec;17(8):401-5. | |||||
| REF 29 | Tranylcypromine: new perspectives on an "old" drug. Eur Arch Psychiatry Clin Neurosci. 2006 Aug;256(5):268-73. | |||||
| REF 30 | Inhibition of rat brain monoamine oxidase activities by psoralen and isopsoralen: implications for the treatment of affective disorders. Pharmacol Toxicol. 2001 Feb;88(2):75-80. | |||||
| REF 31 | Binding of beta-carbolines at imidazoline I2 receptors: a structure-affinity investigation. Bioorg Med Chem Lett. 2004 Feb 23;14(4):999-1002. | |||||
| REF 32 | Reversible inhibitors of monoamine oxidase-A (RIMAs): robust, reversible inhibition of human brain MAO-A by CX157. Neuropsychopharmacology. 2010 Feb;35(3):623-31. | |||||
| REF 33 | Proposed structural basis of interaction of piperine and related compounds with monoamine oxidases. Bioorg Med Chem Lett. 2010 Jan 15;20(2):537-40. | |||||
| REF 34 | LSD1 inhibitors: a patent review (2010-2015).Expert Opin Ther Pat. 2016 May;26(5):565-80. | |||||
| REF 35 | Novel monoamine oxidase inhibitors: a patent review (2012 - 2014).Expert Opin Ther Pat. 2015 Jan;25(1):91-110. | |||||
| REF 36 | The Protein Data Bank. Nucleic Acids Res. 2000 Jan 1;28(1):235-42. | |||||
| REF 37 | A patent review of butyrylcholinesterase inhibitors and reactivators 2010-2017.Expert Opin Ther Pat. 2018 Jun;28(6):455-465. | |||||
| REF 38 | Befloxatone, a new reversible and selective monoamine oxidase-A inhibitor. II. Pharmacological profile. J Pharmacol Exp Ther. 1996 Apr;277(1):265-77. | |||||
| REF 39 | Preclinical profiles of the novel reversible MAO-A inhibitors, moclobemide and brofaromine, in comparison with irreversible MAO inhibitors. J Neural Transm Suppl. 1989;28:5-20. | |||||
| REF 40 | Mechanisms of spinal reflex depressant effects of CS-722, a newly synthesized centrally acting muscle relaxant, in spinal rats. Neuropharmacology. 1992 Sep;31(9):949-54. | |||||
| REF 41 | Stereospecific oxidation of the (S)-enantiomer of RS-8359, a selective and reversible monoamine oxidase A (MAO-A) inhibitor, by aldehyde oxidase. Xenobiotica. 2005 Jun;35(6):561-73. | |||||
| REF 42 | Preclinical and early clinical studies with BW 1370U87, a reversible competitive monoamine oxidase-A inhibitor. Clin Neuropharmacol. 1993;16 Suppl 2:S25-33. | |||||
| REF 43 | Species differences and mechanism of the epimerization of a new MAO-A inhibitor. Xenobiotica. 1998 Mar;28(3):269-80. | |||||
| REF 44 | 2-Arylthiomorpholine derivatives as potent and selective monoamine oxidase B inhibitors. Bioorg Med Chem. 2010 Feb 15;18(4):1388-95. | |||||
| REF 45 | Naphthylisopropylamine and N-benzylamphetamine derivatives as monoamine oxidase inhibitors. Bioorg Med Chem. 2009 Mar 15;17(6):2452-60. | |||||
| REF 46 | Discovery of a novel class of potent coumarin monoamine oxidase B inhibitors: development and biopharmacological profiling of 7-[(3-chlorobenzyl)ox... J Med Chem. 2009 Nov 12;52(21):6685-706. | |||||
| REF 47 | Inhibition of monoamine oxidase by (E)-styrylisatin analogues. Bioorg Med Chem Lett. 2009 May 1;19(9):2509-13. | |||||
| REF 48 | Novel dual inhibitors of AChE and MAO derived from hydroxy aminoindan and phenethylamine as potential treatment for Alzheimer's disease. J Med Chem. 2002 Nov 21;45(24):5260-79. | |||||
| REF 49 | New pyrrole inhibitors of monoamine oxidase: synthesis, biological evaluation, and structural determinants of MAO-A and MAO-B selectivity. J Med Chem. 2007 Mar 8;50(5):922-31. | |||||
| REF 50 | 3-(1H-Pyrrol-1-yl)-2-oxazolidinones as reversible, highly potent, and selective inhibitors of monoamine oxidase type A. J Med Chem. 2002 Mar 14;45(6):1180-3. | |||||
| REF 51 | Human and rat monoamine oxidase-A are differentially inhibited by (S)-4-alkylthioamphetamine derivatives: insights from molecular modeling studies. Bioorg Med Chem. 2007 Aug 1;15(15):5198-206. | |||||
| REF 52 | Pyrazino[1,2-a]indoles as novel high-affinity and selective imidazoline I(2) receptor ligands. Bioorg Med Chem Lett. 2004 Feb 23;14(4):1003-5. | |||||
| REF 53 | Synthesis of 3-benzyl-2-substituted quinoxalines as novel monoamine oxidase A inhibitors. Bioorg Med Chem Lett. 2006 Mar 15;16(6):1753-6. | |||||
| REF 54 | Synthesis, semipreparative HPLC separation, biological evaluation, and 3D-QSAR of hydrazothiazole derivatives as human monoamine oxidase B inhibitors. Bioorg Med Chem. 2010 Jul 15;18(14):5063-70. | |||||
| REF 55 | Ultrasound promoted synthesis of 2-imidazolines in water: a greener approach toward monoamine oxidase inhibitors. Bioorg Med Chem Lett. 2009 Jan 15;19(2):546-9. | |||||
| REF 56 | 3-[5-(4,5-dihydro-1H-imidazol-2-yl)-furan-2-yl]phenylamine (Amifuraline), a promising reversible and selective peripheral MAO-A inhibitor. J Med Chem. 2006 Sep 7;49(18):5578-86. | |||||
| REF 57 | Probes for imidazoline binding sites: synthesis and evaluation of a selective, irreversible I2 ligand. Bioorg Med Chem Lett. 2000 Mar 20;10(6):605-7. | |||||
| REF 58 | Design of novel nicotinamides as potent and selective monoamine oxidase a inhibitors. Bioorg Med Chem. 2010 Feb 15;18(4):1659-64. | |||||
| REF 59 | Binding of an imidazopyridoindole at imidazoline I2 receptors. Bioorg Med Chem Lett. 2004 Jan 19;14(2):527-9. | |||||
| REF 60 | Synthesis, molecular modeling, and selective inhibitory activity against human monoamine oxidases of 3-carboxamido-7-substituted coumarins. J Med Chem. 2009 Apr 9;52(7):1935-42. | |||||
| REF 61 | Synthesis and monoamine oxidase inhibitory activity of new pyridazine-, pyrimidine- and 1,2,4-triazine-containing tricyclic derivatives. J Med Chem. 2007 Nov 1;50(22):5364-71. | |||||
| REF 62 | Quantitative structure-activity relationship and complex network approach to monoamine oxidase A and B inhibitors. J Med Chem. 2008 Nov 13;51(21):6740-51. | |||||
| REF 63 | Inhibition of monoamine oxidase by indole and benzofuran derivatives. Eur J Med Chem. 2010 Oct;45(10):4458-66. | |||||
| REF 64 | A QSAR model for in silico screening of MAO-A inhibitors. Prediction, synthesis, and biological assay of novel coumarins. J Med Chem. 2006 Feb 9;49(3):1149-56. | |||||
| REF 65 | New pyrazoline bearing 4(3H)-quinazolinone inhibitors of monoamine oxidase: synthesis, biological evaluation, and structural determinants of MAO-A ... Bioorg Med Chem. 2009 Jan 15;17(2):675-89. | |||||
| REF 66 | Structures of human monoamine oxidase B complexes with selective noncovalent inhibitors: safinamide and coumarin analogs. J Med Chem. 2007 Nov 15;50(23):5848-52. | |||||
| REF 67 | Inhibition of monoamine oxidase by 8-benzyloxycaffeine analogues. Bioorg Med Chem. 2010 Feb;18(3):1018-28. | |||||
| REF 68 | Differential behavioural and neurochemical effects of para-methoxyamphetamine and 3,4-methylenedioxymethamphetamine in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 2000 Aug;24(6):955-77. | |||||
| REF 69 | Fluorinated phenylcyclopropylamines. Part 5: Effects of electron-withdrawing or -donating aryl substituents on the inhibition of monoamine oxidases... Bioorg Med Chem. 2008 Aug 1;16(15):7148-66. | |||||
| REF 70 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 2489). | |||||
| REF 71 | How many drug targets are there Nat Rev Drug Discov. 2006 Dec;5(12):993-6. | |||||
| REF 72 | Fluorinated phenylcyclopropylamines. 2. Effects of aromatic ring substitution and of absolute configuration on inhibition of microbial tyramine oxi... J Med Chem. 2004 Nov 18;47(24):5860-71. | |||||
| REF 73 | Design, synthesis, and biological evaluation of semicarbazide-sensitive amine oxidase (SSAO) inhibitors with anti-inflammatory activity. J Med Chem. 2006 Apr 6;49(7):2166-73. | |||||
| REF 74 | Synthesis, structure-activity relationships and molecular modeling studies of new indole inhibitors of monoamine oxidases A and B. Bioorg Med Chem. 2008 Nov 15;16(22):9729-40. | |||||
| REF 75 | Solid-phase synthesis and insights into structure-activity relationships of safinamide analogues as potent and selective inhibitors of type B monoa... J Med Chem. 2007 Oct 4;50(20):4909-16. | |||||
| REF 76 | Synthesis and preclinical evaluations of 2-(2-fluorophenyl)-6,7-methylenedioxyquinolin-4-one monosodium phosphate (CHM-1-P-Na) as a potent antitumo... J Med Chem. 2010 Feb 25;53(4):1616-26. | |||||
| REF 77 | Three-dimensional structure of human monoamine oxidase A (MAO A): relation to the structures of rat MAO A and human MAO B. Proc Natl Acad Sci U S A. 2005 Sep 6;102(36):12684-9. | |||||
| REF 78 | Structure of human monoamine oxidase A at 2.2-A resolution: the control of opening the entry for substrates/inhibitors. Proc Natl Acad Sci U S A. 2008 Apr 15;105(15):5739-44. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

