Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T42000
(Former ID: TTDS00447)
|
|||||
| Target Name |
Interleukin-1 beta (IL1B)
|
|||||
| Synonyms |
IL1F2; IL-1beta; IL-1 beta; Catabolin
Click to Show/Hide
|
|||||
| Gene Name |
IL1B
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 4 Target-related Diseases | + | ||||
| 1 | Mineral excesses [ICD-11: 5B91] | |||||
| 2 | Monogenic autoinflammatory syndrome [ICD-11: 4A60] | |||||
| 3 | Osteoarthritis [ICD-11: FA00-FA05] | |||||
| 4 | Rheumatoid arthritis [ICD-11: FA20] | |||||
| Function |
Initially discovered as the major endogenous pyrogen, induces prostaglandin synthesis, neutrophil influx and activation, T-cell activation and cytokine production, B-cell activation and antibody production, and fibroblast proliferation and collagen production. Promotes Th17 differentiation of T-cells. Synergizes with IL12/interleukin-12 to induce IFNG synthesis from T-helper 1 (Th1) cells. Potent proinflammatory cytokine.
Click to Show/Hide
|
|||||
| BioChemical Class |
Cytokine: interleukin
|
|||||
| UniProt ID | ||||||
| Sequence |
MAEVPELASEMMAYYSGNEDDLFFEADGPKQMKCSFQDLDLCPLDGGIQLRISDHHYSKG
FRQAASVVVAMDKLRKMLVPCPQTFQENDLSTFFPFIFEEEPIFFDTWDNEAYVHDAPVR SLNCTLRDSQQKSLVMSGPYELKALHLQGQDMEQQVVFSMSFVQGEESNDKIPVALGLKE KNLYLSCVLKDDKPTLQLESVDPKNYPKKKMEKRFVFNKIEINNKLEFESAQFPNWYIST SQAENMPVFLGGTKGGQDITDFTMQFVSS Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| ADReCS ID | BADD_A03633 ; BADD_A04013 ; BADD_A05078 | |||||
| HIT2.0 ID | T64BTV | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 4 Approved Drugs | + | ||||
| 1 | Canakinumab | Drug Info | Approved | Rheumatoid arthritis | [2], [3] | |
| 2 | Gallium nitrate | Drug Info | Approved | Hypercalcaemia | [4] | |
| 3 | Glucosamine | Drug Info | Approved | Osteoarthritis | [5], [6] | |
| 4 | Rilonacept | Drug Info | Approved | Arthritis | [7], [8] | |
| Clinical Trial Drug(s) | [+] 5 Clinical Trial Drugs | + | ||||
| 1 | XOMA 052 | Drug Info | Phase 3 | Type-2 diabetes | [9] | |
| 2 | ABT-981 | Drug Info | Phase 2 | Osteoarthritis | [11], [12] | |
| 3 | LY-2189102 | Drug Info | Phase 2 | Cardiovascular disease | [13] | |
| 4 | CYT-013-IL1bQb | Drug Info | Phase 1 | Inflammation | [14] | |
| 5 | TT-301 | Drug Info | Phase 1 | Alzheimer disease | [15] | |
| Discontinued Drug(s) | [+] 1 Discontinued Drugs | + | ||||
| 1 | CDP-484 | Drug Info | Discontinued in Phase 1/2 | Immune System disease | [16] | |
| Preclinical Drug(s) | [+] 1 Preclinical Drugs | + | ||||
| 1 | Celastrol | Drug Info | Preclinical | Motor neurone disease | [17] | |
| Mode of Action | [+] 3 Modes of Action | + | ||||
| Inhibitor | [+] 5 Inhibitor drugs | + | ||||
| 1 | Gallium nitrate | Drug Info | [1] | |||
| 2 | Glucosamine | Drug Info | [19] | |||
| 3 | Diacerein | Drug Info | [20] | |||
| 4 | TT-301 | Drug Info | [25] | |||
| 5 | CDP-484 | Drug Info | [26] | |||
| Modulator | [+] 3 Modulator drugs | + | ||||
| 1 | Rilonacept | Drug Info | [8] | |||
| 2 | ABT-981 | Drug Info | [22] | |||
| 3 | DVD-Ig | Drug Info | [24] | |||
| Suppressor | [+] 1 Suppressor drugs | + | ||||
| 1 | Celastrol | Drug Info | [17] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: N-{2-[4-(AMINOSULFONYL)PHENYL]ETHYL}ACETAMIDE | Ligand Info | |||||
| Structure Description | PanDDA analysis group deposition INTERLEUKIN-1 BETA -- Fragment Z30857828 in complex with INTERLEUKIN-1 BETA | PDB:5R8C | ||||
| Method | X-ray diffraction | Resolution | 1.54 Å | Mutation | No | [27] |
| PDB Sequence |
APVRSLNCTL
10 RDSQQKSLVM20 SGPYELKALH30 LQGQDMEQQV40 VFSMSFVQGE50 ESNDKIPVAL 60 GLKEKNLYLS70 CVLKDDKPTL80 QLESVDPKNY90 PKKKMEKRFV100 FNKIEINNKL 110 EFESAQFPNW120 YISTSQAENM130 PVFLGGTKGG140 QDITDFTMQF150 VS |
|||||
|
|
||||||
| Ligand Name: 1-methyl-N-{[(2S)-oxolan-2-yl]methyl}-1H-pyrazole-3-carboxamide | Ligand Info | |||||
| Structure Description | PanDDA analysis group deposition INTERLEUKIN-1 BETA -- Fragment Z2643472210 in complex with INTERLEUKIN-1 BETA | PDB:5R8Q | ||||
| Method | X-ray diffraction | Resolution | 1.23 Å | Mutation | No | [27] |
| PDB Sequence |
APVRSLNCTL
10 RDSQQKSLVM20 SGPYELKALH30 LQGQDMEQQV40 VFSMSFVQGE50 ESNDKIPVAL 60 GLKEKNLYLS70 CVLKDDKPTL80 QLESVDPKNY90 PKKKMEKRFV100 FNKIEINNKL 110 EFESAQFPNW120 YISTSQAENM130 PVFLGGTKGG140 QDITDFTMQF150 VS |
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| MAPK signaling pathway | hsa04010 | Affiliated Target |
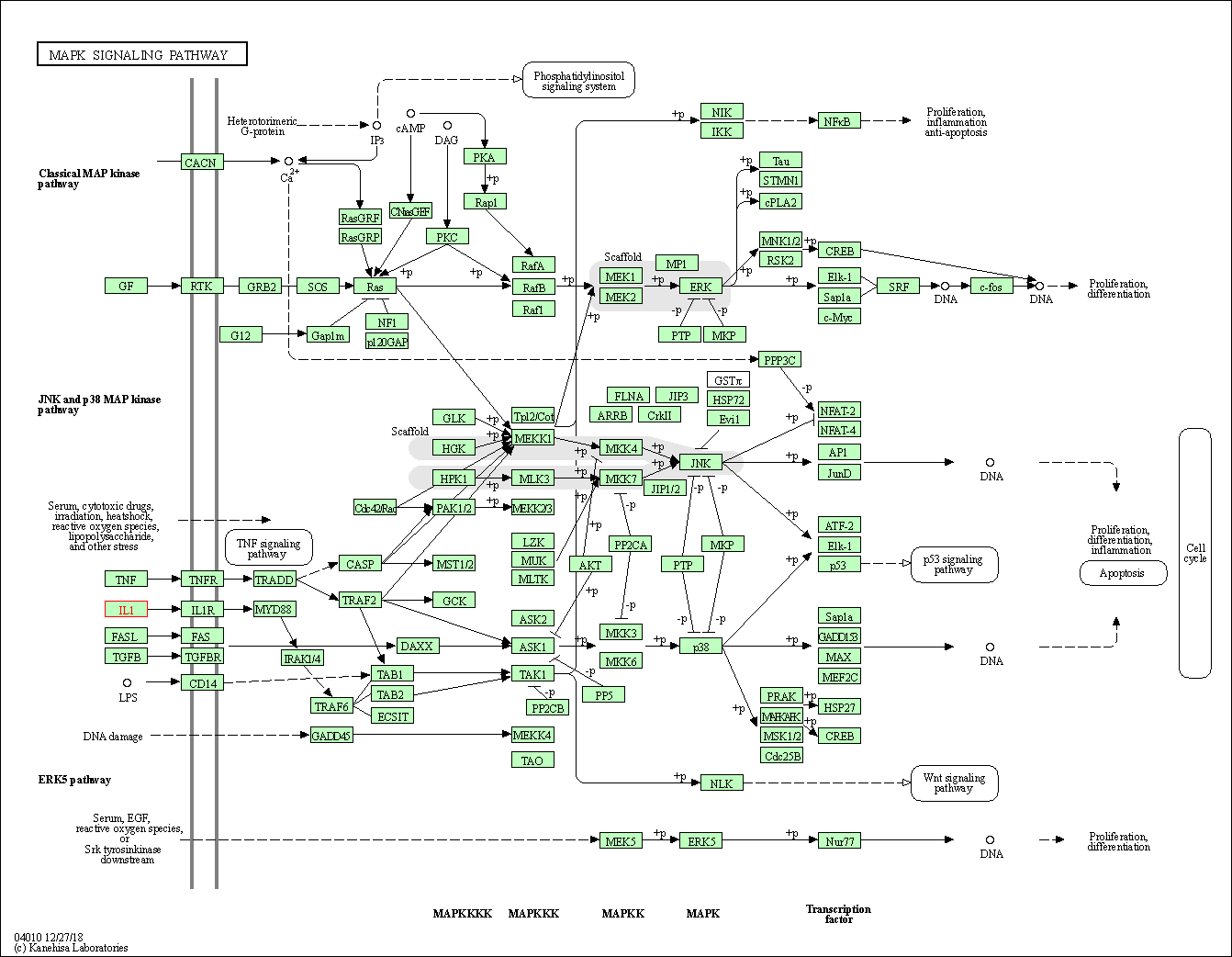
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Cytokine-cytokine receptor interaction | hsa04060 | Affiliated Target |
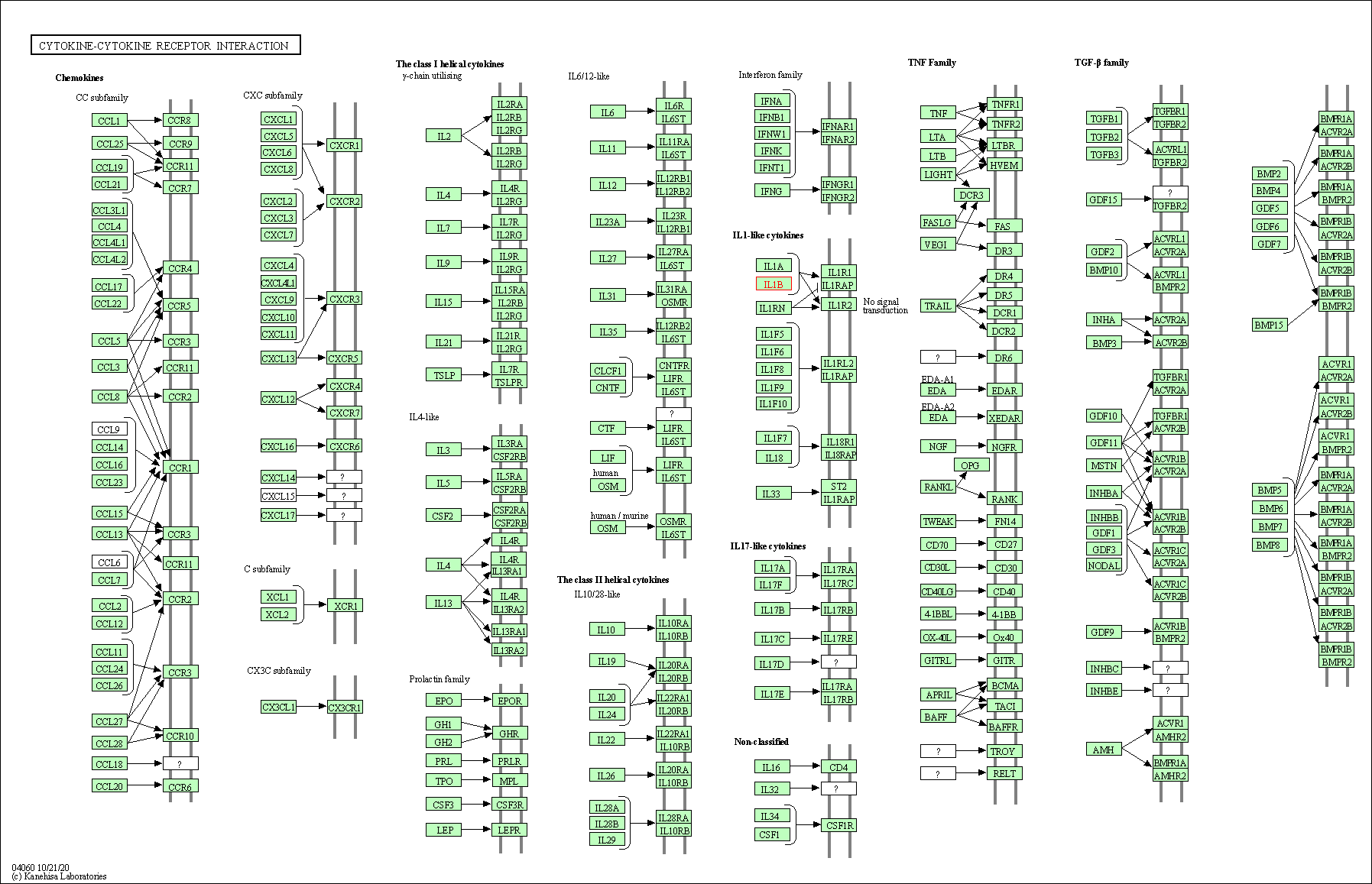
|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
| NF-kappa B signaling pathway | hsa04064 | Affiliated Target |
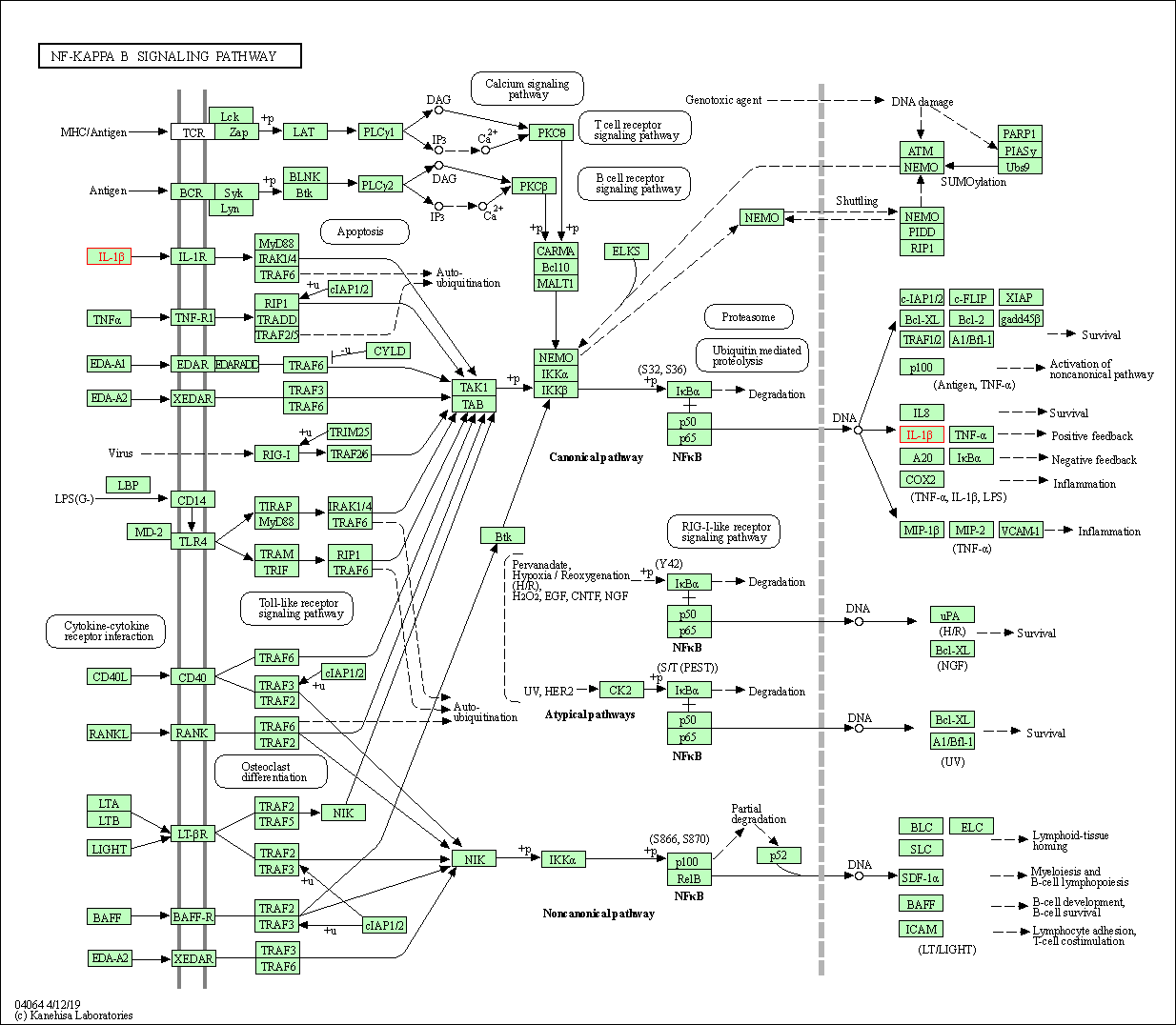
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Necroptosis | hsa04217 | Affiliated Target |
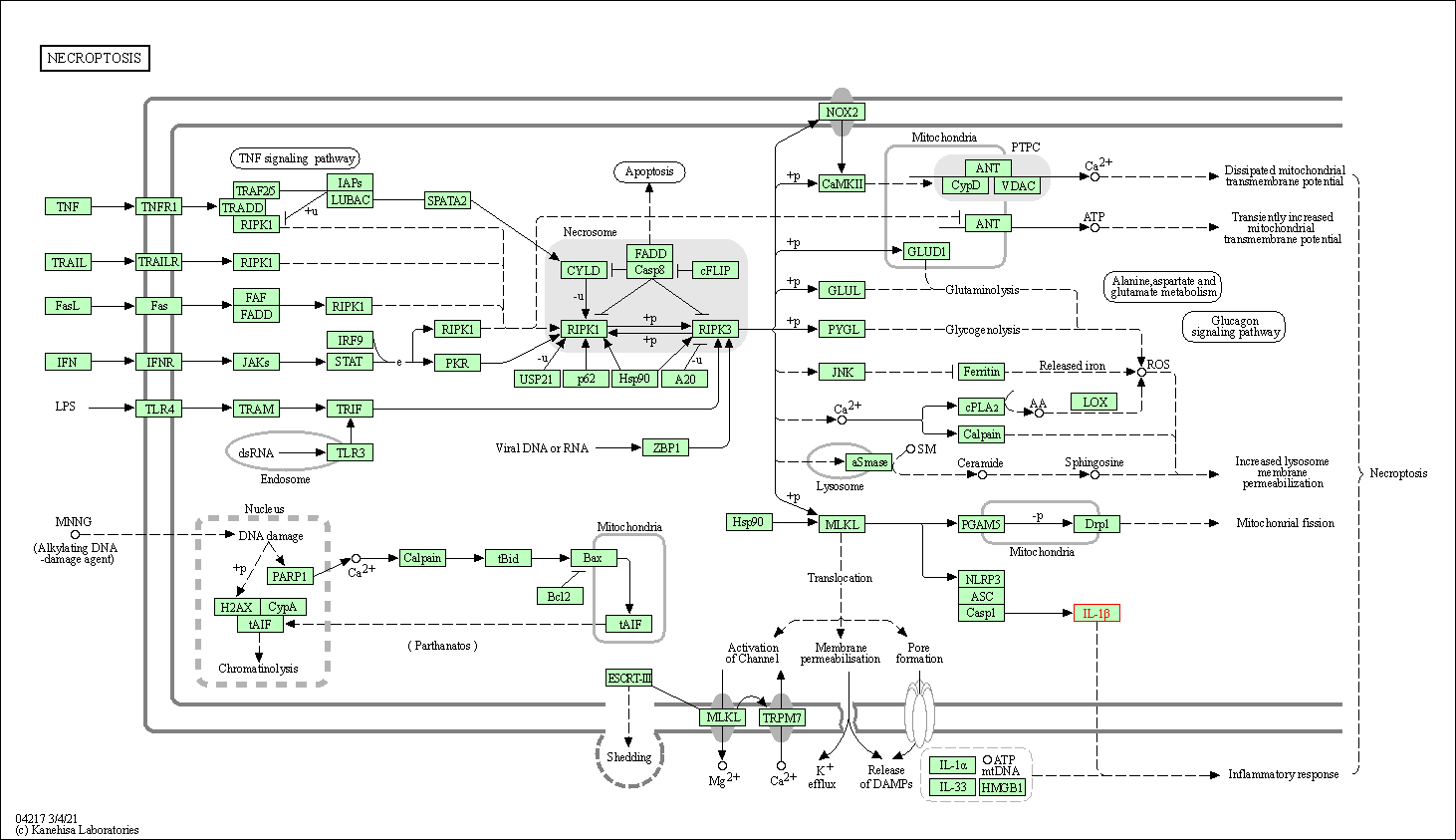
|
| Class: Cellular Processes => Cell growth and death | Pathway Hierarchy | ||
| Osteoclast differentiation | hsa04380 | Affiliated Target |
|
| Class: Organismal Systems => Development and regeneration | Pathway Hierarchy | ||
| Toll-like receptor signaling pathway | hsa04620 | Affiliated Target |
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| NOD-like receptor signaling pathway | hsa04621 | Affiliated Target |
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Cytosolic DNA-sensing pathway | hsa04623 | Affiliated Target |
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| C-type lectin receptor signaling pathway | hsa04625 | Affiliated Target |
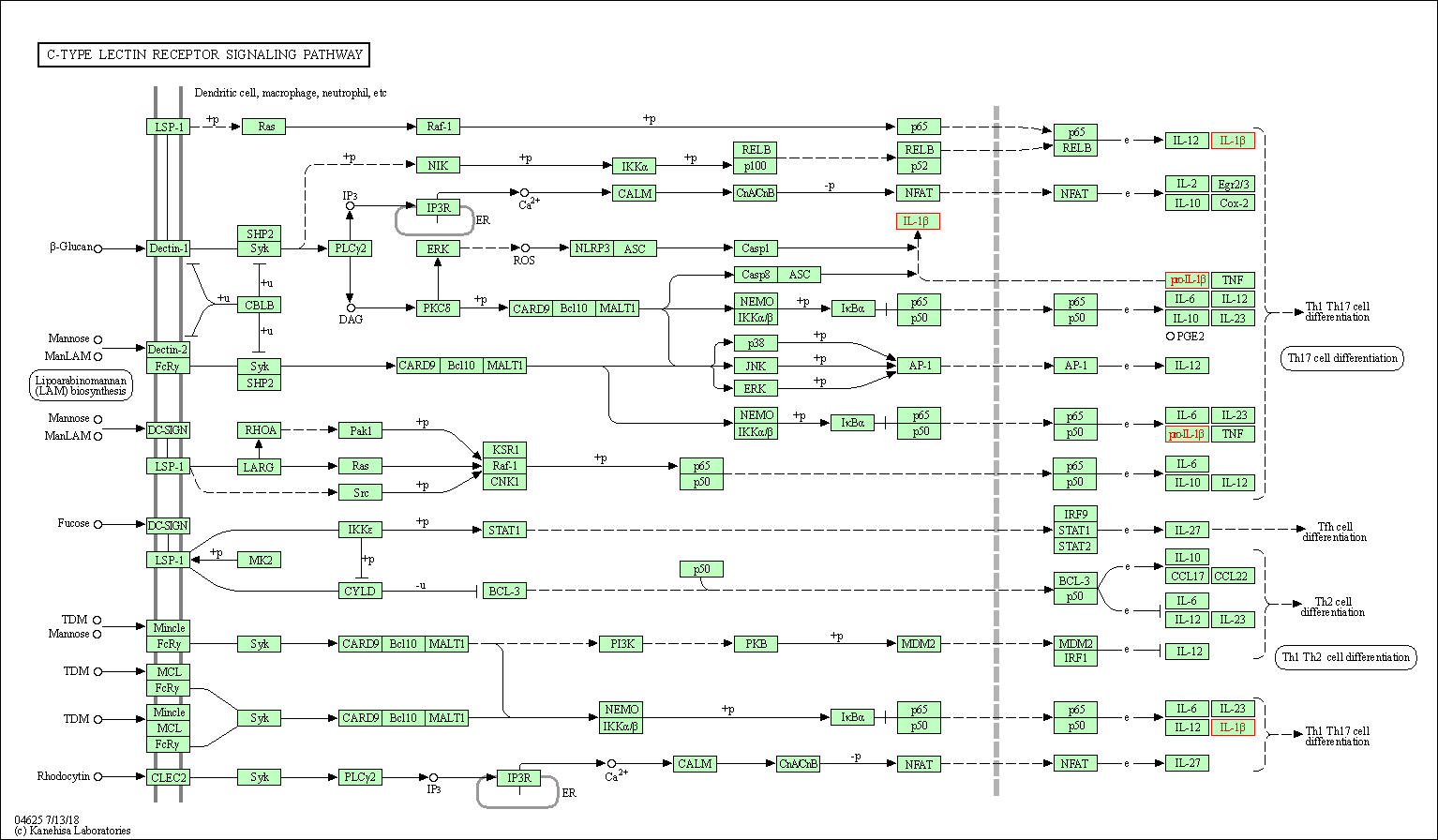
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Hematopoietic cell lineage | hsa04640 | Affiliated Target |
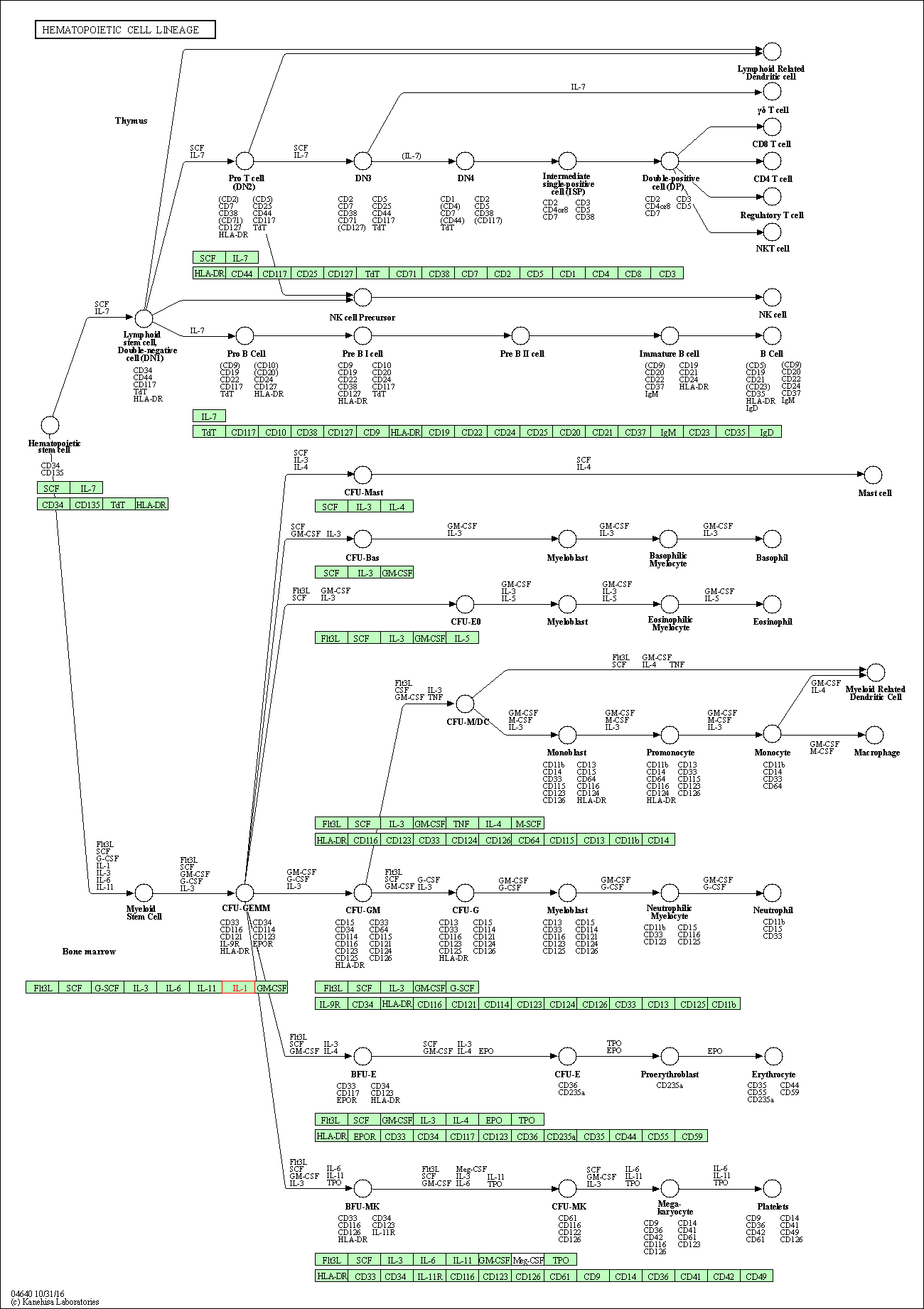
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| IL-17 signaling pathway | hsa04657 | Affiliated Target |
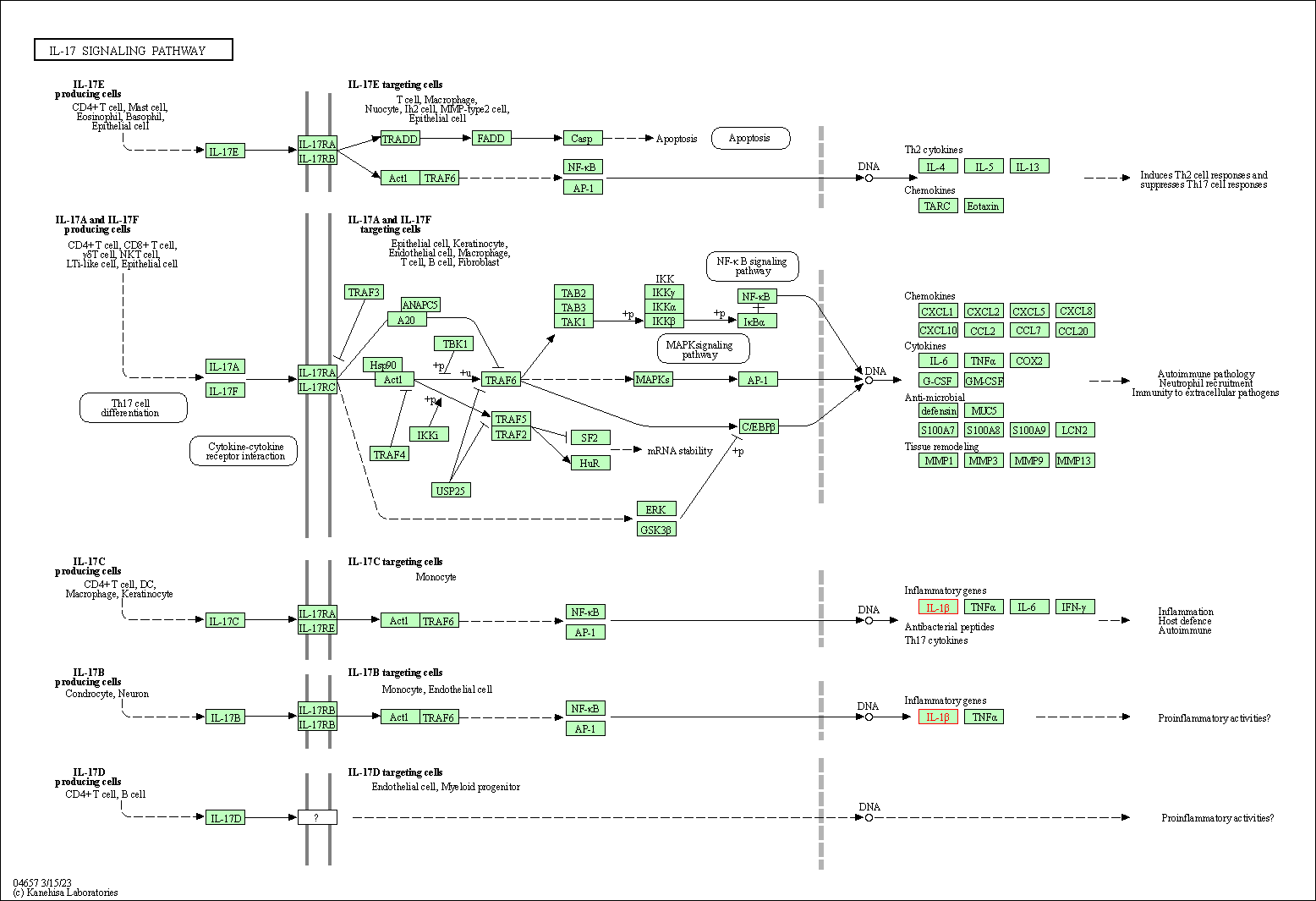
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Th17 cell differentiation | hsa04659 | Affiliated Target |
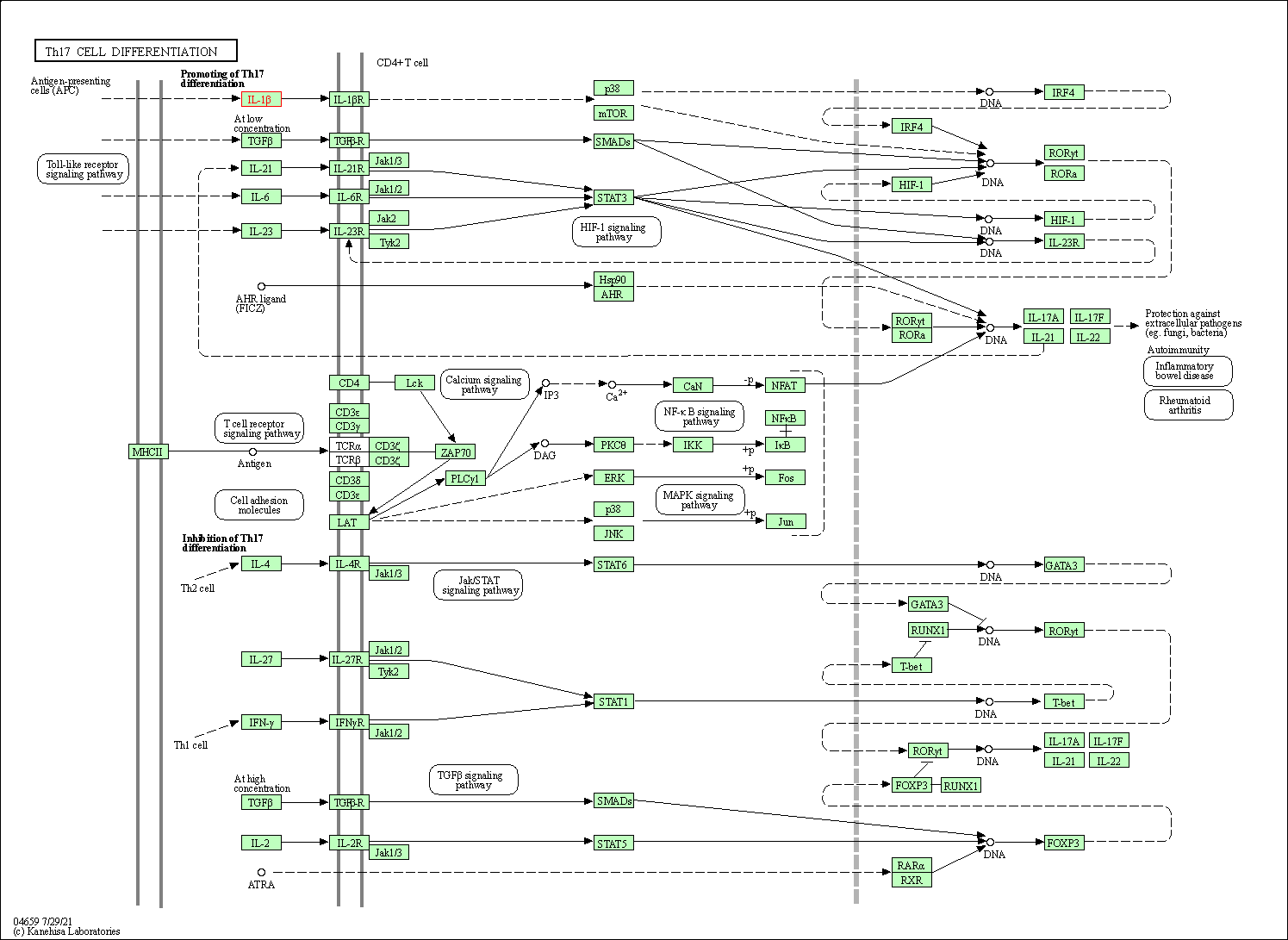
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| TNF signaling pathway | hsa04668 | Affiliated Target |
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Inflammatory mediator regulation of TRP channels | hsa04750 | Affiliated Target |
|
| Class: Organismal Systems => Sensory system | Pathway Hierarchy | ||
| Click to Show/Hide the Information of Affiliated Human Pathways | |||
| Degree | 46 | Degree centrality | 4.94E-03 | Betweenness centrality | 1.51E-03 |
|---|---|---|---|---|---|
| Closeness centrality | 2.41E-01 | Radiality | 1.42E+01 | Clustering coefficient | 2.31E-01 |
| Neighborhood connectivity | 2.93E+01 | Topological coefficient | 5.87E-02 | Eccentricity | 11 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target-regulating Transcription Factors | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) | ||||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Elimination of arthritis pain and inflammation for over 2 years with a single 90 min, topical 14% gallium nitrate treatment: case reports and revie... Med Hypotheses. 2005;65(6):1136-41. | |||||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6773). | |||||
| REF 3 | Hughes B: 2009 FDA drug approvals. Nat Rev Drug Discov. 2010 Feb;9(2):89-92. | |||||
| REF 4 | Drug information of Gallium nitrate, 2008. eduDrugs. | |||||
| REF 5 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4535). | |||||
| REF 6 | Drug information of Glucosamine, 2008. eduDrugs. | |||||
| REF 7 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6790). | |||||
| REF 8 | 2008 FDA drug approvals. Nat Rev Drug Discov. 2009 Feb;8(2):93-6. | |||||
| REF 9 | ClinicalTrials.gov (NCT02258867) Efficacy and Safety Study of Gevokizumab to Treat Behcet's Disease Uveitis. U.S. National Institutes of Health. | |||||
| REF 10 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 11 | ClinicalTrials.gov (NCT02087904) A Phase 2a Study Evaluating the Safety, Efficacy, and Pharmacodynamic Effects of ABT-981 in Patients With Knee Osteoarthritis | |||||
| REF 12 | ClinicalTrials.gov (NCT02384538) A Phase 2a Study Evaluating the Safety and Efficacy of ABT-981 in Patients With Erosive Hand Osteoarthritis | |||||
| REF 13 | ClinicalTrials.gov (NCT00942188) A Study of LY2189102 in Patients With Type 2 Diabetes. U.S. National Institutes of Health. | |||||
| REF 14 | ClinicalTrials.gov (NCT00924105) Safety, Tolerability and Pharmacodynamics of CYT013-IL1bQb in Patients With Type 2 Diabetes. U.S. National Institutes of Health. | |||||
| REF 15 | ClinicalTrials.gov (NCT01357421) Effects of TT301 on Cytokine Levels Post Endotoxin Challenge. U.S. National Institutes of Health. | |||||
| REF 16 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800016757) | |||||
| REF 17 | Emerging disease-modifying therapies for the treatment of motor neuron disease/amyotropic lateral sclerosis. Expert Opin Emerg Drugs. 2007 May;12(2):229-52. | |||||
| REF 18 | Emerging drugs for rheumatoid arthritis. Expert Opin Emerg Drugs. 2008 Mar;13(1):175-96. | |||||
| REF 19 | Glucosamine inhibits IL-1beta-mediated IL-8 production in prostate cancer cells by MAPK attenuation. J Cell Biochem. 2009 Oct 1;108(2):489-98. | |||||
| REF 20 | Non-surgical treatment of osteoarthritis of large joints - new aspects. Wien Med Wochenschr. 2009;159(3-4):76-86. | |||||
| REF 21 | Gevokizumab, an anti-IL-1beta mAb for the potential treatment of type 1 and 2 diabetes, rheumatoid arthritis and cardiovascular disease. Curr Opin Mol Ther. 2010 Dec;12(6):755-69. | |||||
| REF 22 | Generation and characterization of ABT-981, a dual variable domain immunoglobulin (DVD-Ig(TM)) molecule that specifically and potently neutralizes both IL-1alpha and IL-1beta. MAbs. 2015;7(3):605-19. | |||||
| REF 23 | Double-blind, randomized study evaluating the glycemic and anti-inflammatory effects of subcutaneous LY2189102, a neutralizing IL-1beta antibody, in patients with type 2 diabetes. Diabetes Care. 2013Aug;36(8):2239-46. | |||||
| REF 24 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 2623). | |||||
| REF 25 | A Swell in the Armamentarium of Antiepileptic Drug Targets. Epilepsy Curr. 2011 Nov-Dec; 11(6): 172-176. | |||||
| REF 26 | Biological targets for therapeutic interventions in COPD: clinical potential. Int J Chron Obstruct Pulmon Dis. 2006 September; 1(3): 321-334. | |||||
| REF 27 | Mining the PDB for Tractable Cases Where X-ray Crystallography Combined with Fragment Screens Can Be Used to Systematically Design Protein-Protein Inhibitors: Two Test Cases Illustrated by IL1beta-IL1R and p38Alpha-TAB1 Complexes. J Med Chem. 2020 Jul 23;63(14):7559-7568. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

