Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T76369
(Former ID: TTDR00274)
|
|||||
| Target Name |
Liver carboxylesterase (CES1)
|
|||||
| Synonyms |
Serine esterase 1; Monocyte/macrophage serine esterase; Human carboxylesterase 1; HMSE; HCE1; CES1; Brain carboxylesterase hBr1; Acyl coenzyme A:cholesterol acyltransferase
Click to Show/Hide
|
|||||
| Gene Name |
CES1
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 2 Target-related Diseases | + | ||||
| 1 | Peroxisomal disease [ICD-11: 5C57] | |||||
| 2 | Synthesis disorder [ICD-11: 5C52-5C59] | |||||
| Function |
Involved in the detoxification of xenobiotics and in the activation of ester and amide prodrugs. Hydrolyzes aromatic and aliphatic esters, but has no catalytic activity toward amides or a fatty acyl coa ester.
Click to Show/Hide
|
|||||
| BioChemical Class |
Carboxylic ester hydrolase
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 3.1.1.1
|
|||||
| Sequence |
MWLRAFILATLSASAAWGHPSSPPVVDTVHGKVLGKFVSLEGFAQPVAIFLGIPFAKPPL
GPLRFTPPQPAEPWSFVKNATSYPPMCTQDPKAGQLLSELFTNRKENIPLKLSEDCLYLN IYTPADLTKKNRLPVMVWIHGGGLMVGAASTYDGLALAAHENVVVVTIQYRLGIWGFFST GDEHSRGNWGHLDQVAALRWVQDNIASFGGNPGSVTIFGESAGGESVSVLVLSPLAKNLF HRAISESGVALTSVLVKKGDVKPLAEQIAITAGCKTTTSAVMVHCLRQKTEEELLETTLK MKFLSLDLQGDPRESQPLLGTVIDGMLLLKTPEELQAERNFHTVPYMVGINKQEFGWLIP MQLMSYPLSEGQLDQKTAMSLLWKSYPLVCIAKELIPEATEKYLGGTDDTVKKKDLFLDL IADVMFGVPSVIVARNHRDAGAPTYMYEFQYRPSFSSDMKPKTVIGDHGDELFSVFGAPF LKEGASEEEIRLSKMVMKFWANFARNGNPNGEGLPHWPEYNQKEGYLQIGANTQAAQKLK DKEVAFWTNLFAKKAVEKPPQTEHIEL Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | PDB | ||||
| ADReCS ID | BADD_A00333 | |||||
| HIT2.0 ID | T59DDI | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 1 Approved Drugs | + | ||||
| 1 | Cholic Acid | Drug Info | Approved | Synthesis disorder | [2] | |
| Clinical Trial Drug(s) | [+] 3 Clinical Trial Drugs | + | ||||
| 1 | PACTIMIBE | Drug Info | Phase 2/3 | Arteriosclerosis | [3] | |
| 2 | K-604 | Drug Info | Phase 2 | Arteriosclerosis | [5] | |
| 3 | GR148672X | Drug Info | Clinical trial | Acute lymphoblastic leukaemia | [6] | |
| Discontinued Drug(s) | [+] 20 Discontinued Drugs | + | ||||
| 1 | Avasimibe | Drug Info | Discontinued in Phase 3 | Peripheral vascular disease | [7] | |
| 2 | CI-976 | Drug Info | Discontinued in Phase 2 | Hyperlipidaemia | [9] | |
| 3 | CL-283796 | Drug Info | Discontinued in Phase 2 | Hyperlipidaemia | [10] | |
| 4 | E-5324 | Drug Info | Discontinued in Phase 2 | Hyperlipidaemia | [11] | |
| 5 | RP-64477 | Drug Info | Discontinued in Phase 2 | Hyperlipidaemia | [12] | |
| 6 | 447C88 | Drug Info | Discontinued in Phase 1 | Hyperlipidaemia | [13] | |
| 7 | CL-277082 | Drug Info | Discontinued in Phase 1 | Arteriosclerosis | [14] | |
| 8 | F-1394 | Drug Info | Discontinued in Phase 1 | Arteriosclerosis | [15] | |
| 9 | YM-17E | Drug Info | Discontinued in Phase 1 | Hyperlipidaemia | [16] | |
| 10 | YM-750 | Drug Info | Discontinued in Phase 1 | Hyperlipidaemia | [17] | |
| 11 | CEB-925 | Drug Info | Terminated | Hypercholesterolaemia | [19] | |
| 12 | CI-999 | Drug Info | Terminated | Arteriosclerosis | [20] | |
| 13 | DuP-129 | Drug Info | Terminated | Hypercholesterolaemia | [21] | |
| 14 | FR-129169 | Drug Info | Terminated | Arteriosclerosis | [22] | |
| 15 | FR-145237 | Drug Info | Terminated | Arteriosclerosis | [23] | |
| 16 | Lecimibide | Drug Info | Terminated | Hyperlipidaemia | [24] | |
| 17 | NTE-122 | Drug Info | Terminated | Arteriosclerosis | [25] | |
| 18 | RP-70676 | Drug Info | Terminated | Hyperlipidaemia | [26] | |
| 19 | RP-73163 | Drug Info | Terminated | Arteriosclerosis | [27] | |
| 20 | TEI-6522 | Drug Info | Terminated | Arteriosclerosis | [28] | |
| Preclinical Drug(s) | [+] 1 Preclinical Drugs | + | ||||
| 1 | HL-004 | Drug Info | Preclinical | Arteriosclerosis | [18] | |
| Mode of Action | [+] 2 Modes of Action | + | ||||
| Inhibitor | [+] 101 Inhibitor drugs | + | ||||
| 1 | Cholic Acid | Drug Info | [1] | |||
| 2 | PACTIMIBE | Drug Info | [29] | |||
| 3 | K-604 | Drug Info | [30] | |||
| 4 | GR148672X | Drug Info | [31] | |||
| 5 | Avasimibe | Drug Info | [32] | |||
| 6 | CI-976 | Drug Info | [33] | |||
| 7 | YM-750 | Drug Info | [42] | |||
| 8 | CEB-925 | Drug Info | [44] | |||
| 9 | CI-999 | Drug Info | [45] | |||
| 10 | DuP-129 | Drug Info | [31] | |||
| 11 | NTE-122 | Drug Info | [49] | |||
| 12 | (E)-Octadec-9-enoic acid phenylamide | Drug Info | [53] | |||
| 13 | 1,1,1-trifluoro-3-(hexylsulfinyl)propan-2-one | Drug Info | [54] | |||
| 14 | 1,1,1-trifluoro-3-(hexylsulfonyl)propan-2-one | Drug Info | [54] | |||
| 15 | 1,1,1-trifluoro-3-(octylsulfinyl)propan-2-one | Drug Info | [54] | |||
| 16 | 1,1,1-trifluoro-3-(octylsulfonyl)propan-2-one | Drug Info | [54] | |||
| 17 | 1,1,1-trifluoro-3-(octylthio)propan-2-one | Drug Info | [54] | |||
| 18 | 1,1,1-trifluorododecan-2-one | Drug Info | [54] | |||
| 19 | 1,10-phenanthroline-5,6-dione | Drug Info | [55] | |||
| 20 | 1,2-bis(2,3,4-trifluorophenyl)-2-hydroxyethanone | Drug Info | [56] | |||
| 21 | 1,2-bis(2,3,4-trifluorophenyl)ethane-1,2-dione | Drug Info | [56] | |||
| 22 | 1,2-bis(2,3,5-trifluorophenyl)-2-hydroxyethanone | Drug Info | [56] | |||
| 23 | 1,2-bis(2,3,5-trifluorophenyl)ethane-1,2-dione | Drug Info | [56] | |||
| 24 | 1,2-bis(2,3,6-trifluorophenyl)ethane-1,2-dione | Drug Info | [56] | |||
| 25 | 1,2-bis(2,3-difluorophenyl)-2-hydroxyethanone | Drug Info | [56] | |||
| 26 | 1,2-bis(2,3-fluorophenyl)ethane-1,2-dione | Drug Info | [56] | |||
| 27 | 1,2-bis(2,4-difluorophenyl)ethane-1,2-dione | Drug Info | [56] | |||
| 28 | 1,2-bis(2,5-difluorophenyl)-2-hydroxyethanone | Drug Info | [56] | |||
| 29 | 1,2-bis(2,5-difluorophenyl)ethane-1,2-dione | Drug Info | [56] | |||
| 30 | 1,2-bis(2,6-difluorophenyl)-2-hydroxyethanone | Drug Info | [56] | |||
| 31 | 1,2-bis(2,6-difluorophenyl)ethane-1,2-dione | Drug Info | [56] | |||
| 32 | 1,2-bis(2-fluorophenyl)-2-hydroxyethanone | Drug Info | [56] | |||
| 33 | 1,2-bis(2-fluorophenyl)ethane-1,2-dione | Drug Info | [56] | |||
| 34 | 1,2-bis(3,4,5-trifluorophenyl)-2-hydroxyethanone | Drug Info | [56] | |||
| 35 | 1,2-bis(3,4,5-trifluorophenyl)ethane-1,2-dione | Drug Info | [56] | |||
| 36 | 1,2-bis(3,4-difluorophenyl)-2-hydroxyethanone | Drug Info | [56] | |||
| 37 | 1,2-bis(3,4-difluorophenyl)ethane-1,2-dione | Drug Info | [56] | |||
| 38 | 1,2-bis(3,5-difluorophenyl)-2-hydroxyethanone | Drug Info | [56] | |||
| 39 | 1,2-bis(3,5-difluorophenyl)ethane-1,2-dione | Drug Info | [56] | |||
| 40 | 1,2-bis(3-fluorophenyl)-2-hydroxyethanon | Drug Info | [56] | |||
| 41 | 1,2-bis(4-fluorophenyl)ethane-1,2-dione | Drug Info | [56] | |||
| 42 | 1,2-Bis-(2-chloro-phenyl)-ethane-1,2-dione | Drug Info | [57] | |||
| 43 | 1,2-Bis-(3-methoxy-phenyl)-ethane-1,2-dione | Drug Info | [57] | |||
| 44 | 1,2-Bis-(4-bromo-phenyl)-ethane-1,2-dione | Drug Info | [57] | |||
| 45 | 1,2-Bis-(4-chloro-phenyl)-ethane-1,2-dione | Drug Info | [57] | |||
| 46 | 1,2-Di-naphthalen-2-yl-ethane-1,2-dione | Drug Info | [58] | |||
| 47 | 1,2-dicyclohexylethane-1,2-dione | Drug Info | [55] | |||
| 48 | 1,2-indanedione | Drug Info | [55] | |||
| 49 | 1-(2-bromoethyl)-1H-indole-2,3-dione | Drug Info | [59] | |||
| 50 | 1-(2-iodoethyl)-1H-indole-2,3-dione | Drug Info | [59] | |||
| 51 | 1-(3,4-dichlorobenzyl)-1H-indole-2,3-dione | Drug Info | [59] | |||
| 52 | 1-(3,4-Dimethyl-phenyl)-2-phenyl-ethane-1,2-dione | Drug Info | [57] | |||
| 53 | 1-(4-Chloro-phenyl)-2-p-tolyl-ethane-1,2-dione | Drug Info | [57] | |||
| 54 | 1-(4-Chloro-phenyl)-2-phenyl-ethane-1,2-dione | Drug Info | [57] | |||
| 55 | 1-(4-chlorobenzyl)-1H-indole-2,3-dione | Drug Info | [59] | |||
| 56 | 1-(4-Methoxy-phenyl)-2-phenyl-ethane-1,2-dione | Drug Info | [57] | |||
| 57 | 1-(4-Nitro-phenyl)-2-phenyl-ethane-1,2-dione | Drug Info | [57] | |||
| 58 | 1-benzyl-1H-indole-2,3-dione | Drug Info | [59] | |||
| 59 | 1-butyryl-1H-indole-2,3-dione | Drug Info | [59] | |||
| 60 | 1-dodecyl-1H-indole-2,3-dione | Drug Info | [59] | |||
| 61 | 1-phenyl-1H-indole-2,3-dione | Drug Info | [59] | |||
| 62 | 1-Phenyl-2-p-tolyl-ethane-1,2-dione | Drug Info | [57] | |||
| 63 | 1-Phenyl-propane-1,2-dione | Drug Info | [57] | |||
| 64 | 1-propionyl-1H-indole-2,3-dione | Drug Info | [59] | |||
| 65 | 11,12-dihydro-dibenzo[a,e]cyclooctene-5,6-dione | Drug Info | [55] | |||
| 66 | 2,2-Dimethoxy-1,2-diphenyl-ethanone | Drug Info | [57] | |||
| 67 | 2,2-dimethyl-3-methyleneheptadecane | Drug Info | [54] | |||
| 68 | 2-methoxy-3,4-methylenedioxybenzophenone | Drug Info | [60] | |||
| 69 | 3,4,5,6-Tetrachloro-[1,2]benzoquinone | Drug Info | [57] | |||
| 70 | 3-(butylsulfinyl)-1,1,1-trifluoropropan-2-one | Drug Info | [54] | |||
| 71 | 3-(butylthio)-1,1,1-trifluoropropan-2-one | Drug Info | [54] | |||
| 72 | 3-(decylsulfinyl)-1,1,1-trifluoropropan-2-one | Drug Info | [54] | |||
| 73 | 3-(decylthio)-1,1,1-trifluoropropan-2-one | Drug Info | [54] | |||
| 74 | 3-(dodecylsulfinyl)-1,1,1-trifluoropropan-2-one | Drug Info | [54] | |||
| 75 | 3-(dodecylsulfonyl)-1,1,1-trifluoropropan-2-one | Drug Info | [54] | |||
| 76 | 4,6-dichloro-1H-indole-2,3-dione | Drug Info | [59] | |||
| 77 | 4,7-dichloro-1H-indole-2,3-dione | Drug Info | [59] | |||
| 78 | 4-(2-Oxo-2-phenyl-acetyl)-benzoic acid | Drug Info | [57] | |||
| 79 | 4-chloro-1H-indole-2,3-dione | Drug Info | [59] | |||
| 80 | 4-chloro-7-methyl-1H-indole-2,3-dione | Drug Info | [59] | |||
| 81 | 4-Piperidino-Piperidine | Drug Info | [61] | |||
| 82 | 5,7-dichloro-1H-indole-2,3-dione | Drug Info | [59] | |||
| 83 | 5-chloro-1H-indole-2,3-dione | Drug Info | [59] | |||
| 84 | 6-bromo-5-methyl-1H-indole-2,3-dione | Drug Info | [59] | |||
| 85 | 7-(trifluoromethyl)-1H-indole-2,3-dione | Drug Info | [59] | |||
| 86 | Acenanthrene-9,10-dione | Drug Info | [55] | |||
| 87 | ACENAPHTHOQUINONE | Drug Info | [55] | |||
| 88 | Alpha-D-Mannose | Drug Info | [1] | |||
| 89 | CHLORANIL | Drug Info | [57] | |||
| 90 | Dibutyl 2,2,2-trifluoro-1-phenylethyl phosphate | Drug Info | [62] | |||
| 91 | Diethyl 2,2,2-trifluoro-1-phenylethyl phosphate | Drug Info | [62] | |||
| 92 | Heptane-2,3-dione | Drug Info | [57] | |||
| 93 | N-Methylnaloxonium | Drug Info | [63] | |||
| 94 | NSC-23180 | Drug Info | [55] | |||
| 95 | O-Sialic Acid | Drug Info | [61] | |||
| 96 | Oleic acid anilide | Drug Info | [60] | |||
| 97 | Phenanthrene-9,10-dione | Drug Info | [55] | |||
| 98 | PYRIPYROPENE A | Drug Info | [53] | |||
| 99 | Thenoyltrifluoroacetone | Drug Info | [65] | |||
| 100 | Thieno[3,2-e][1]benzothiophene-4,5-dione | Drug Info | [55] | |||
| 101 | VULM-1457 | Drug Info | [31] | |||
| Modulator | [+] 15 Modulator drugs | + | ||||
| 1 | CL-283796 | Drug Info | [34], [35] | |||
| 2 | E-5324 | Drug Info | [36] | |||
| 3 | RP-64477 | Drug Info | [37] | |||
| 4 | 447C88 | Drug Info | [38] | |||
| 5 | CL-277082 | Drug Info | [39] | |||
| 6 | F-1394 | Drug Info | [40] | |||
| 7 | YM-17E | Drug Info | [41] | |||
| 8 | HL-004 | Drug Info | [43] | |||
| 9 | FR-129169 | Drug Info | [46] | |||
| 10 | FR-145237 | Drug Info | [47] | |||
| 11 | Lecimibide | Drug Info | [48] | |||
| 12 | RP-70676 | Drug Info | [50] | |||
| 13 | RP-73163 | Drug Info | [51] | |||
| 14 | TEI-6522 | Drug Info | [52] | |||
| 15 | SMP-797 | Drug Info | [64] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Tacrine | Ligand Info | |||||
| Structure Description | Crystal Structure of Human Liver Carboxylesterase in complex with tacrine | PDB:1MX1 | ||||
| Method | X-ray diffraction | Resolution | 2.40 Å | Mutation | No | [66] |
| PDB Sequence |
SSPPVVDTVH
1030 GKVLGKFVSL1040 EGFAQPVAIF1050 LGIPFAKPPL1060 GPLRFTPPQP1070 AEPWSFVKNA 1080 TSYPPMCTQD1090 PKAGQLLSEL1100 FTNRKENIPL1110 KLSEDCLYLN1120 IYTPADLTKK 1130 NRLPVMVWIH1140 GGGLMVGAAS1150 TYDGLALAAH1160 ENVVVVTIQY1170 RLGIWGFFST 1180 GDEHSRGNWG1190 HLDQVAALRW1200 VQDNIASFGG1210 NPGSVTIFGE1220 SAGGESVSVL 1230 VLSPLAKNLF1240 HRAISESGVA1250 LTSVLVKKGD1260 VKPLAEQIAI1270 TAGCKTTTSA 1280 VMVHCLRQKT1290 EEELLETTLK1300 MKFLSLDLQG1310 DPRESQPLLG1320 TVIDGMLLLK 1330 TPEELQAERN1340 FHTVPYMVGI1350 NKQEFGWLIP1360 MLMSYPLSEG1371 QLDQKTAMSL 1381 LWKSYPLVCI1391 AKELIPEATE1401 KYLGGTDDTV1411 KKKDLFLDLI1421 ADVMFGVPSV 1431 IVARNHRDAG1441 APTYMYEFQY1451 RPSFSSDMKP1461 KTVIGDHGDE1471 LFSVFGAPFL 1481 KEGASEEEIR1491 LSKMVMKFWA1501 NFARNGNPNG1511 EGLPHWPEYN1521 QKEGYLQIGA 1531 NTQAAQKLKD1541 KEVAFWTNLF1551 AK
|
|||||
|
|
LEU1097
3.384
PHE1101
3.532
GLY1141
4.634
GLY1142
3.815
GLY1143
4.042
GLU1220
4.561
SER1221
2.855
ALA1222
4.771
THR1252
4.892
VAL1254
3.694
|
|||||
| Ligand Name: Cholic acid | Ligand Info | |||||
| Structure Description | Crystal structure of human carboxylesterase in complex with cholate and palmitate | PDB:2DQY | ||||
| Method | X-ray diffraction | Resolution | 3.00 Å | Mutation | No | [67] |
| PDB Sequence |
SSPPVVDTVH
1030 GKVLGKFVSL1040 EGFAQPVAIF1050 LGIPFAKPPL1060 GPLRFTPPQP1070 AEPWSFVKNA 1080 TSYPPMCTQD1090 PKAGQLLSEL1100 FTNRKENIPL1110 KLSEDCLYLN1120 IYTPADLTKK 1130 NRLPVMVWIH1140 GGGLMVGAAS1150 TYDGLALAAH1160 ENVVVVTIQY1170 RLGIWGFFST 1180 GDEHSRGNWG1190 HLDQVAALRW1200 VQDNIASFGG1210 NPGSVTIFGE1220 SAGGESVSVL 1230 VLSPLAKNLF1240 HRAISESGVA1250 LTSVLVKKGD1260 VKPLAEQIAI1270 TAGCKTTTSA 1280 VMVHCLRQKT1290 EEELLETTLK1300 MKFLSLDLQG1310 DPRESQPLLG1320 TVIDGMLLLK 1330 TPEELQAERN1340 FHTVPYMVGI1350 NKQEFGWLIP1360 MLMSYPLSEG1371 QLDQKTAMSL 1381 LWKSYPLVCI1391 AKELIPEATE1401 KYLGGTDDTV1411 KKKDLFLDLI1421 ADVMFGVPSV 1431 IVARNHRDAG1441 APTYMYEFQY1451 RPSFSSDMKP1461 KTVIGDHGDE1471 LFSVFGAPFL 1481 KEGASEEEIR1491 LSKMVMKFWA1501 NFARNGNPNG1511 EGLPHWPEYN1521 QKEGYLQIGA 1531 NTQAAQKLKD1541 KEVAFWTNLF1551 AK
|
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Drug metabolism - other enzymes | hsa00983 | Affiliated Target |
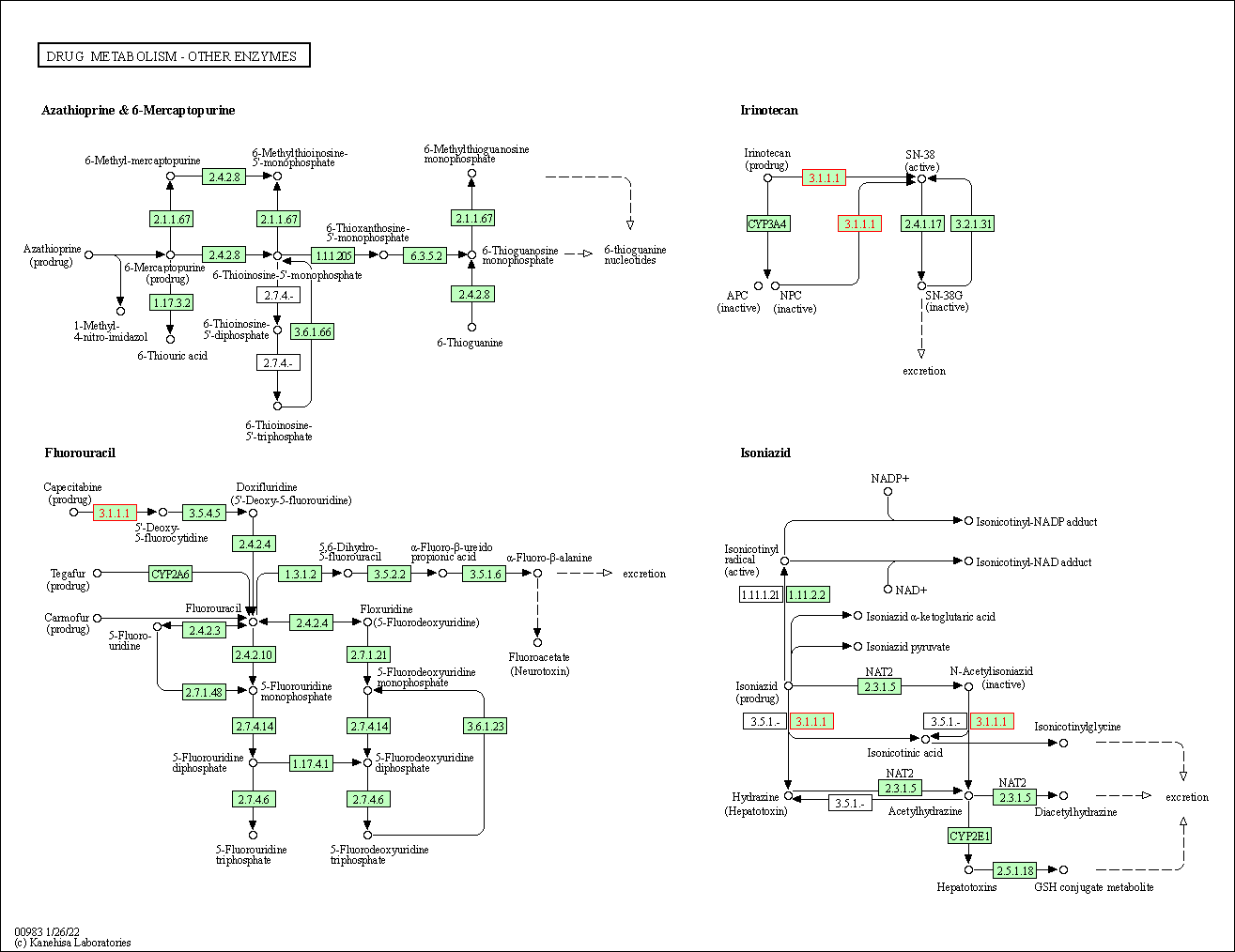
|
| Class: Metabolism => Xenobiotics biodegradation and metabolism | Pathway Hierarchy | ||
| Degree | 2 | Degree centrality | 2.15E-04 | Betweenness centrality | 4.05E-04 |
|---|---|---|---|---|---|
| Closeness centrality | 1.60E-01 | Radiality | 1.24E+01 | Clustering coefficient | 0.00E+00 |
| Neighborhood connectivity | 1.10E+01 | Topological coefficient | 5.00E-01 | Eccentricity | 12 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) | ||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 2 KEGG Pathways | + | ||||
| 1 | Drug metabolism - other enzymes | |||||
| 2 | Metabolic pathways | |||||
| PID Pathway | [+] 1 PID Pathways | + | ||||
| 1 | E2F transcription factor network | |||||
| WikiPathways | [+] 6 WikiPathways | + | ||||
| 1 | NRF2 pathway | |||||
| 2 | Nuclear Receptors Meta-Pathway | |||||
| 3 | Heroin metabolism | |||||
| 4 | Irinotecan Pathway | |||||
| 5 | Fluoropyrimidine Activity | |||||
| 6 | Phase I biotransformations, non P450 | |||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | How many drug targets are there Nat Rev Drug Discov. 2006 Dec;5(12):993-6. | |||||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 609). | |||||
| REF 3 | ClinicalTrials.gov (NCT00151788) Efficacy and Safety of the ACAT Inhibitor CS-505 (Pactimibe) for Reducing the Progression of Carotid Artery Disease. This Study is Also Known as CAPTIVATE.. U.S. National Institutes of Health. | |||||
| REF 4 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800003592) | |||||
| REF 5 | ClinicalTrials.gov (NCT00851500) A Trial of the Safety and Efficacy of K-604 for the Treatment of Atherosclerosis. U.S. National Institutes of Health. | |||||
| REF 6 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6701). | |||||
| REF 7 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800008778) | |||||
| REF 8 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800011031) | |||||
| REF 9 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800001196) | |||||
| REF 10 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800004604) | |||||
| REF 11 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800004427) | |||||
| REF 12 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002301) | |||||
| REF 13 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002257) | |||||
| REF 14 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800001648) | |||||
| REF 15 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800003109) | |||||
| REF 16 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002618) | |||||
| REF 17 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800003090) | |||||
| REF 18 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800033616) | |||||
| REF 19 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800010079) | |||||
| REF 20 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800010990) | |||||
| REF 21 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800003587) | |||||
| REF 22 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002683) | |||||
| REF 23 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800006465) | |||||
| REF 24 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002283) | |||||
| REF 25 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800008195) | |||||
| REF 26 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002876) | |||||
| REF 27 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800003588) | |||||
| REF 28 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800004501) | |||||
| REF 29 | Novel indoline-based acyl-CoA:cholesterol acyltransferase inhibitor with antiperoxidative activity: improvement of physicochemical properties and b... J Med Chem. 2008 Aug 14;51(15):4823-33. | |||||
| REF 30 | A selective ACAT-1 inhibitor, K-604, suppresses fatty streak lesions in fat-fed hamsters without affecting plasma cholesterol levels. Atherosclerosis. 2007 Apr;191(2):290-7. | |||||
| REF 31 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 2592). | |||||
| REF 32 | New advances in lipid-modifying therapies for reducing cardiovascular risk. Cardiology. 2002;97(2):59-66. | |||||
| REF 33 | Acyl-coenzyme A:cholesterol-acyltransferase (ACAT) inhibitors modulate monocyte adhesion to aortic endothelial cells. Atherosclerosis. 1995 Jan 6;112(1):7-17. | |||||
| REF 34 | ACAT inhibitors CL 283,546 and CL 283,796 reduce LDL cholesterol without affecting cholesterol absorption in African green monkeys. J Lipid Res. 1995 Jun;36(6):1199-210. | |||||
| REF 35 | Therapeutic target database update 2012: a resource for facilitating target-oriented drug discovery. Nucleic Acids Res. 2012 Jan;40(Database issue):D1128-36. | |||||
| REF 36 | Effect of the acyl-CoA:cholesterol acyltransferase inhibitor, E5324, on experimental atherosclerosis in rabbits. Atherosclerosis. 1994 Jun;107(2):187-201. | |||||
| REF 37 | RP 64477: a potent inhibitor of acyl-coenzyme A:cholesterol O-acyltransferase with low systemic bioavailability. Biochem Pharmacol. 1996 Feb 23;51(4):413-21. | |||||
| REF 38 | The tolerability, pharmacokinetics and lack of effect on plasma cholesterol of 447C88, an AcylCoA: Cholesterol Acyl Transferase (ACAT) inhibitor with low bioavailability, in healthy volunteers. Eur JClin Pharmacol. 1995;49(3):243-9. | |||||
| REF 39 | CL 277,082: a novel inhibitor of ACAT-catalyzed cholesterol esterification and cholesterol absorption. J Lipid Res. 1989 May;30(5):681-90. | |||||
| REF 40 | ACAT inhibitor F-1394 prevents intimal hyperplasia induced by balloon injury in rabbits. J Lipid Res. 2001 Apr;42(4):480-8. | |||||
| REF 41 | Pharmacological properties of YM17E, an acyl-CoA:cholesterol acyltransferase inhibitor, and diarrheal effect in beagle dogs. Jpn J Pharmacol. 1997 Jan;73(1):41-50. | |||||
| REF 42 | Effects of an anti-oxidative ACAT inhibitor on apoptosis/necrosis and cholesterol accumulation under oxidative stress in THP-1 cell-derived foam ce... Life Sci. 2008 Jan 2;82(1-2):79-84. | |||||
| REF 43 | ACAT inhibitor HL-004 accelerates the regression of hypercholesterolemia in stroke-prone spontaneously hypertensive rats (SHRSP): stimulation of bile acid production by HL-004. Atherosclerosis. 1997 Aug;133(1):97-104. | |||||
| REF 44 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800010079) | |||||
| REF 45 | Inhibitors of acyl-CoA:cholesterol O-acyltransferase (ACAT) as hypocholesterolemic agents: synthesis and structure-activity relationships of novel series of sulfonamides, acylphosphonamides and acylphosphoramidates. Bioorg Med Chem Lett. 1998 Feb 3;8(3):289-94. | |||||
| REF 46 | Plasma cholesterol reducing effect of FR129169, a novel acyl-CoA:cholesterol acyltransferase inhibitor, in the rat. Jpn J Pharmacol. 1996 Jan;70(1):35-41. | |||||
| REF 47 | Effect of FR145237, a novel ACAT inhibitor, on atherogenesis in cholesterol-fed and WHHL rabbits. Evidence for a direct effect on the arterial wall. Biochim Biophys Acta. 1995 Dec 7;1259(3):254-60. | |||||
| REF 48 | Effect of the acyl-CoA:cholesterol acyltransferase inhibitor DuP 128 on cholesterol absorption and serum cholesterol in humans. Clin Pharmacol Ther. 1994 Jul;56(1):65-74. | |||||
| REF 49 | Cholesterol-lowering effects of NTE-122, a novel acyl-CoA:cholesterol acyltransferase (ACAT) inhibitor, on cholesterol diet-fed rats and rabbits. Jpn J Pharmacol. 1998 Nov;78(3):355-64. | |||||
| REF 50 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002876) | |||||
| REF 51 | Hypolipidaemic properties of a potent and bioavailable alkylsulphinyl-diphenylimidazole ACAT inhibitor (RP 73163) in animals fed diets low in cholesterol. Biochem Pharmacol. 1996 Oct 25;52(8):1177-86. | |||||
| REF 52 | Potent inhibitors of acyl-CoA:cholesterol acyltransferase. Structure-activity relationships of novel N-(4-oxochroman-8-yl)amides. J Med Chem. 1995 Aug 4;38(16):3174-86. | |||||
| REF 53 | Acyl-CoA: cholesterol acyltransferase inhibitory activities of fatty acid amides isolated from Mylabris phalerate Pallas. Bioorg Med Chem Lett. 2004 Aug 16;14(16):4277-80. | |||||
| REF 54 | Influence of sulfur oxidation state and steric bulk upon trifluoromethyl ketone (TFK) binding kinetics to carboxylesterases and fatty acid amide hy... Bioorg Med Chem. 2008 Feb 15;16(4):2114-30. | |||||
| REF 55 | Planarity and constraint of the carbonyl groups in 1,2-diones are determinants for selective inhibition of human carboxylesterase 1. J Med Chem. 2007 Nov 15;50(23):5727-34. | |||||
| REF 56 | Analysis of the inhibition of mammalian carboxylesterases by novel fluorobenzoins and fluorobenzils. Bioorg Med Chem. 2007 Jun 1;15(11):3801-17. | |||||
| REF 57 | Identification and characterization of novel benzil (diphenylethane-1,2-dione) analogues as inhibitors of mammalian carboxylesterases. J Med Chem. 2005 Apr 21;48(8):2906-15. | |||||
| REF 58 | Inhibition of carboxylesterases by benzil (diphenylethane-1,2-dione) and heterocyclic analogues is dependent upon the aromaticity of the ring and t... J Med Chem. 2005 Aug 25;48(17):5543-50. | |||||
| REF 59 | Selective inhibition of carboxylesterases by isatins, indole-2,3-diones. J Med Chem. 2007 Apr 19;50(8):1876-85. | |||||
| REF 60 | Phenolic compounds from the roots of Lindera fruticosa. J Nat Prod. 2006 May;69(5):853-5. | |||||
| REF 61 | The Protein Data Bank. Nucleic Acids Res. 2000 Jan 1;28(1):235-42. | |||||
| REF 62 | Synthesis of organophosphates with fluorine-containing leaving groups as serine esterase inhibitors with potential for Alzheimer disease therapeutics. Bioorg Med Chem Lett. 2009 Oct 1;19(19):5528-30. | |||||
| REF 63 | DrugBank 3.0: a comprehensive resource for 'omics' research on drugs. Nucleic Acids Res. 2011 Jan;39(Database issue):D1035-41. | |||||
| REF 64 | Company report (Dainippon Sumitomo Pharma) | |||||
| REF 65 | Zhang JG, Fariss MW: Thenoyltrifluoroacetone, a potent inhibitor of carboxylesterase activity. Biochem Pharmacol. 2002 Feb 15;63(4):751-4. | |||||
| REF 66 | Crystal structure of human carboxylesterase 1 complexed with the Alzheimer's drug tacrine: from binding promiscuity to selective inhibition. Chem Biol. 2003 Apr;10(4):341-9. | |||||
| REF 67 | Multisite promiscuity in the processing of endogenous substrates by human carboxylesterase 1. J Mol Biol. 2006 Oct 13;363(1):201-14. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

