Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T84631
(Former ID: TTDS00203)
|
|||||
| Target Name |
Coagulation factor Xa (F10)
|
|||||
| Synonyms |
Fxa; Factor Xa; F10; Activated coagulation factor X
Click to Show/Hide
|
|||||
| Gene Name |
F10
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 8 Target-related Diseases | + | ||||
| 1 | Blood-forming organ disease [ICD-11: 3C0Z] | |||||
| 2 | Christmas disease [ICD-11: 3B11] | |||||
| 3 | Coagulation defect [ICD-11: 3B10] | |||||
| 4 | Coronary thrombosis [ICD-11: BA43] | |||||
| 5 | Deep vein thrombosis [ICD-11: BD71] | |||||
| 6 | Supraventricular tachyarrhythmia [ICD-11: BC81] | |||||
| 7 | Thrombosis [ICD-11: DB61-GB90] | |||||
| 8 | Venous thromboembolism [ICD-11: BD72] | |||||
| Function |
Factor Xa is avitamin K-dependent glycoprotein that converts prothrombin to thrombin in the presence of factor Va, calcium and phospholipid during blood clotting.
Click to Show/Hide
|
|||||
| BioChemical Class |
Peptidase
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 3.4.21.6
|
|||||
| Sequence |
MGRPLHLVLLSASLAGLLLLGESLFIRREQANNILARVTRANSFLEEMKKGHLERECMEE
TCSYEEAREVFEDSDKTNEFWNKYKDGDQCETSPCQNQGKCKDGLGEYTCTCLEGFEGKN CELFTRKLCSLDNGDCDQFCHEEQNSVVCSCARGYTLADNGKACIPTGPYPCGKQTLERR KRSVAQATSSSGEAPDSITWKPYDAADLDPTENPFDLLDFNQTQPERGDNNLTRIVGGQE CKDGECPWQALLINEENEGFCGGTILSEFYILTAAHCLYQAKRFKVRVGDRNTEQEEGGE AVHEVEVVIKHNRFTKETYDFDIAVLRLKTPITFRMNVAPACLPERDWAESTLMTQKTGI VSGFGRTHEKGRQSTRLKMLEVPYVDRNSCKLSSSFIITQNMFCAGYDTKQEDACQGDSG GPHVTRFKDTYFVTGIVSWGEGCARKGKYGIYTKVTAFLKWIDRSMKTRGLPKAKSHAPE VITSSPLK Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| ADReCS ID | BADD_A00959 | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 10 Approved Drugs | + | ||||
| 1 | Apixaban | Drug Info | Approved | Thrombosis | [3], [4] | |
| 2 | BETRIXABAN | Drug Info | Approved | Venous thromboembolism | [5], [6] | |
| 3 | Coagulation Factor IX | Drug Info | Approved | Haemophilia B | [7], [8] | |
| 4 | Danaparoid | Drug Info | Approved | Deep venous clot | [9], [10] | |
| 5 | DU-176b | Drug Info | Approved | Atrial fibrillation | [11], [12] | |
| 6 | Emicizumab | Drug Info | Approved | Factor VIII deficiency | [6] | |
| 7 | Fondaparinux sodium | Drug Info | Approved | Venous thrombosis | [13] | |
| 8 | Lmw heparin | Drug Info | Approved | Hematologic disease | [2] | |
| 9 | Nadroparin calcium | Drug Info | Approved | Coagulation defect | [2] | |
| 10 | Rivaroxaban | Drug Info | Approved | Deep vein thrombosis | [14], [15] | |
| Clinical Trial Drug(s) | [+] 11 Clinical Trial Drugs | + | ||||
| 1 | Human coagulation factor X | Drug Info | BLA submitted | Renal cell carcinoma | [16] | |
| 2 | PRT4445 | Drug Info | Phase 3 | Bleeding disorder | [18] | |
| 3 | SSR-126517E | Drug Info | Phase 3 | Thrombosis | [19] | |
| 4 | Antistasin | Drug Info | Phase 2 | Attention deficit hyperactivity disorder | [22] | |
| 5 | GW-813893 | Drug Info | Phase 2 | Thrombosis | [23] | |
| 6 | LY-517717 | Drug Info | Phase 2 | Thrombosis | [24] | |
| 7 | BI-11634 | Drug Info | Phase 1 | Thrombosis | [27] | |
| 8 | EP-42675 | Drug Info | Phase 1 | Thrombosis | [28] | |
| 9 | GCC-4401 | Drug Info | Phase 1 | Thrombosis | [29] | |
| 10 | R-1663 | Drug Info | Phase 1 | Coagulation defect | [30] | |
| 11 | SSR-128428 | Drug Info | Phase 1 | Thrombosis | [31] | |
| Discontinued Drug(s) | [+] 17 Discontinued Drugs | + | ||||
| 1 | Darexaban maleate | Drug Info | Discontinued in Phase 3 | Acute coronary syndrome | [32] | |
| 2 | Idraparinux | Drug Info | Discontinued in Phase 3 | Thrombosis | [33] | |
| 3 | Otamixaban | Drug Info | Discontinued in Phase 3 | Angina pectoris | [34] | |
| 4 | Valspodar | Drug Info | Discontinued in Phase 3 | Acute myeloid leukaemia | [35] | |
| 5 | Octopamine | Drug Info | Discontinued in Phase 2a | Thrombosis | [36], [37] | |
| 6 | DX-9065a | Drug Info | Discontinued in Phase 2 | Angina pectoris | [39] | |
| 7 | PD-348292 | Drug Info | Discontinued in Phase 2 | Thrombosis | [40] | |
| 8 | R-68151 | Drug Info | Discontinued in Phase 2 | Psoriasis vulgaris | [41] | |
| 9 | TAK-442 | Drug Info | Discontinued in Phase 2 | Thrombosis | [42] | |
| 10 | AVE-3247 | Drug Info | Discontinued in Phase 1 | Thrombosis | [43] | |
| 11 | DPC 423 | Drug Info | Discontinued in Phase 1 | Thrombosis | [44] | |
| 12 | EMD-503982 | Drug Info | Discontinued in Phase 1 | Thrombosis | [45] | |
| 13 | JTV-803 | Drug Info | Discontinued in Phase 1 | Thrombosis | [46] | |
| 14 | YM-75466 | Drug Info | Discontinued in Phase 1 | Thrombosis | [47] | |
| 15 | ZD-4927 | Drug Info | Discontinued in Phase 1 | Thrombosis | [48] | |
| 16 | Draculin | Drug Info | Terminated | Thrombosis | [51] | |
| 17 | Hirufaxin | Drug Info | Terminated | Thrombosis | [52] | |
| Preclinical Drug(s) | [+] 1 Preclinical Drugs | + | ||||
| 1 | CS-3030 | Drug Info | Preclinical | Thrombosis | [50] | |
| Mode of Action | [+] 4 Modes of Action | + | ||||
| Inhibitor | [+] 55 Inhibitor drugs | + | ||||
| 1 | Apixaban | Drug Info | [1], [53] | |||
| 2 | Danaparoid | Drug Info | [56] | |||
| 3 | Fondaparinux sodium | Drug Info | [58], [59] | |||
| 4 | MELAGATRAN | Drug Info | [64] | |||
| 5 | SSR-126517E | Drug Info | [62], [63] | |||
| 6 | Antistasin | Drug Info | [65] | |||
| 7 | GW-813893 | Drug Info | [62], [63] | |||
| 8 | LY-517717 | Drug Info | [62], [63] | |||
| 9 | BI-11634 | Drug Info | [62], [63] | |||
| 10 | GCC-4401 | Drug Info | [62], [63] | |||
| 11 | R-1663 | Drug Info | [62], [63] | |||
| 12 | Darexaban maleate | Drug Info | [68] | |||
| 13 | Idraparinux | Drug Info | [69] | |||
| 14 | Otamixaban | Drug Info | [69] | |||
| 15 | Valspodar | Drug Info | [65], [70] | |||
| 16 | Octopamine | Drug Info | [71], [72] | |||
| 17 | DX-9065a | Drug Info | [73] | |||
| 18 | PD-348292 | Drug Info | [1], [74] | |||
| 19 | R-68151 | Drug Info | [75] | |||
| 20 | TAK-442 | Drug Info | [76] | |||
| 21 | AVE-3247 | Drug Info | [62], [63] | |||
| 22 | DPC 423 | Drug Info | [77] | |||
| 23 | EMD-503982 | Drug Info | [62], [63] | |||
| 24 | JTV-803 | Drug Info | [62], [63] | |||
| 25 | YM-75466 | Drug Info | [62], [63] | |||
| 26 | ZD-4927 | Drug Info | [62], [63] | |||
| 27 | CS-3030 | Drug Info | [62], [63] | |||
| 28 | Hirufaxin | Drug Info | [62], [63] | |||
| 29 | YM60828 | Drug Info | [65] | |||
| 30 | 1,2,3,4,6-penta-O-galloyl-beta-D-glucose | Drug Info | [79] | |||
| 31 | 3-chlorophenyl 2-oxo-2H-chromene-3-carboxylate | Drug Info | [80] | |||
| 32 | 4-(4-Benzyloxy-3-methoxy-benzylamino)-benzamidine | Drug Info | [81] | |||
| 33 | 5-desgalloylstachyurin | Drug Info | [79] | |||
| 34 | BMS-269223 | Drug Info | [82] | |||
| 35 | BMS-344577 | Drug Info | [82] | |||
| 36 | BMS-740808 | Drug Info | [83] | |||
| 37 | CASUARIIN | Drug Info | [79] | |||
| 38 | D-Pro-Phe-Arg chloromethyl ketone | Drug Info | [86] | |||
| 39 | Gamma-Carboxy-Glutamic Acid | Drug Info | [87] | |||
| 40 | GC-2107 | Drug Info | [62], [63] | |||
| 41 | Lefaxin | Drug Info | [65] | |||
| 42 | M55113 | Drug Info | [88] | |||
| 43 | Molecule 11 | Drug Info | [89] | |||
| 44 | PhSO2-Gly-(Me-Gly)-Arg-(2-thiazole) | Drug Info | [90] | |||
| 45 | PRT-064445 | Drug Info | [62], [63] | |||
| 46 | RAZAXABAN | Drug Info | [83] | |||
| 47 | SC-83157 | Drug Info | [91] | |||
| 48 | SF303 | Drug Info | [77] | |||
| 49 | SK509 | Drug Info | [77] | |||
| 50 | SK554 | Drug Info | [65] | |||
| 51 | SN429 | Drug Info | [91] | |||
| 52 | Tellimagrandin II | Drug Info | [79] | |||
| 53 | YM-96765 | Drug Info | [92] | |||
| 54 | ZK-810388 | Drug Info | [93] | |||
| 55 | ZK-814048 | Drug Info | [93] | |||
| Modulator | [+] 15 Modulator drugs | + | ||||
| 1 | BETRIXABAN | Drug Info | [54] | |||
| 2 | DU-176b | Drug Info | [57] | |||
| 3 | Emicizumab | Drug Info | [6] | |||
| 4 | Lmw heparin | Drug Info | [13], [60] | |||
| 5 | Nadroparin calcium | Drug Info | [13], [61] | |||
| 6 | Rivaroxaban | Drug Info | [2] | |||
| 7 | Human coagulation factor X | Drug Info | [62], [63] | |||
| 8 | PRT4445 | Drug Info | [62], [63] | |||
| 9 | EP-42675 | Drug Info | [66] | |||
| 10 | SSR-128428 | Drug Info | [67] | |||
| 11 | Draculin | Drug Info | [78] | |||
| 12 | CI-1031 | Drug Info | [84], [85] | |||
| 13 | Recombinant coagulation factors | Drug Info | [62], [63] | |||
| 14 | Recombinant Factor X | Drug Info | [62], [63] | |||
| 15 | ZK-813039 | Drug Info | [93] | |||
| Activator | [+] 1 Activator drugs | + | ||||
| 1 | Coagulation Factor IX | Drug Info | [55] | |||
| Antagonist | [+] 2 Antagonist drugs | + | ||||
| 1 | DT-831j | Drug Info | [62], [63] | |||
| 2 | EP-37 | Drug Info | [62], [63] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Rivaroxaban | Ligand Info | |||||
| Structure Description | Factor Xa in complex with BAY59-7939 | PDB:2W26 | ||||
| Method | X-ray diffraction | Resolution | 2.08 Å | Mutation | No | [94] |
| PDB Sequence |
IVGGQECKDG
25 ECPWQALLIN35 EENEGFCGGT45 ILSEFYILTA55 AHCLYQAKRF64 KVRVGDRNTE 74 QEEGGEAVHE84 VEVVIKHNRF94 TKETYDFDIA104 VLRLKTPITF114 RMNVAPACLP 124 ERDWAESTLM131B TQKTGIVSGF141 GRTHEKGRQS152 TRLKMLEVPY162 VDRNSCKLSS 172 SFIITQNMFC182 AGYDTKQEDA190 CQGDSGGPHV200 TRFKDTYFVT210 GIVSWGEGCA 221 RKGKYGIYTK230 VTAFLKWIDR240 SMKT
|
|||||
|
|
LYS96
3.660
GLU97
3.127
THR98
2.966
TYR99
3.420
PHE174
3.659
ASP189
3.750
ALA190
3.578
CYS191
3.862
GLN192
3.358
SER195
3.956
VAL213
3.754
|
|||||
| Click to View More Binding Site Information of This Target and Ligand Pair | ||||||
| Ligand Name: Apixaban | Ligand Info | |||||
| Structure Description | Factor Xa in Complex with the Inhibitor APIXABAN (BMS-562247) AKA 1-(4-METHOXYPHENYL)-7-OXO-6-(4-(2-OXO-1-PIPERIDINYL)PHENYL)-4,5,6,7-TETRAHYDRO-1H-PYRAZOLO[3, 4-C]PYRIDINE-3-CARBOXAMIDE | PDB:2P16 | ||||
| Method | X-ray diffraction | Resolution | 2.30 Å | Mutation | No | [83] |
| PDB Sequence |
IVGGQECKDG
25 ECPWQALLIN35 EENEGFCGGT45 ILSEFYILTA55 AHCLYQAKRF64 KVRVGDRNTE 74 QEEGGEAVHE84 VEVVIKHNRF94 TKETYDFDIA104 VLRLKTPITF114 RMNVAPACLP 124 ERDWAESTLM131B TQKTGIVSGF141 GRTHEKGRQS152 TRLKMLEVPY162 VDRNSCKLSS 172 SFIITQNMFC182 AGYDTKQEDA190 CQGDSGGPHV200 TRFKDTYFVT210 GIVSWGEGCA 221 RKGKYGIYTK230 VTAFLKWIDR240 SMKT
|
|||||
|
|
LYS96
4.318
GLU97
3.467
THR98
3.370
TYR99
3.770
ARG143
3.755
GLU146
3.058
PHE174
3.487
ASP189
3.590
ALA190
3.321
CYS191
3.394
GLN192
3.197
|
|||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Complement and coagulation cascades | hsa04610 | Affiliated Target |
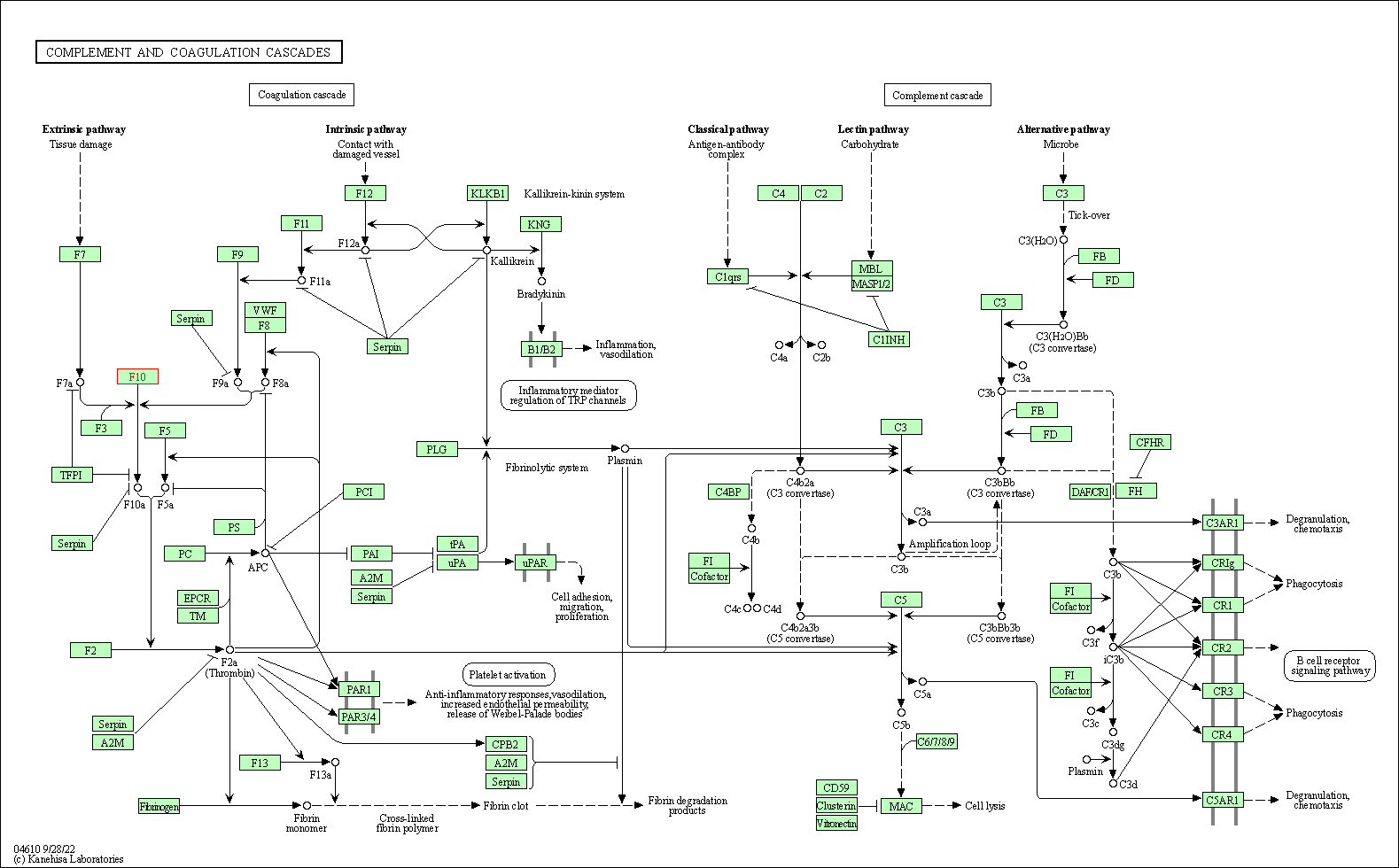
|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Degree | 5 | Degree centrality | 5.37E-04 | Betweenness centrality | 4.08E-04 |
|---|---|---|---|---|---|
| Closeness centrality | 1.74E-01 | Radiality | 1.28E+01 | Clustering coefficient | 3.00E-01 |
| Neighborhood connectivity | 7.80E+00 | Topological coefficient | 3.45E-01 | Eccentricity | 13 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) | ||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 1 KEGG Pathways | + | ||||
| 1 | Complement and coagulation cascades | |||||
| Panther Pathway | [+] 1 Panther Pathways | + | ||||
| 1 | Blood coagulation | |||||
| Pathwhiz Pathway | [+] 1 Pathwhiz Pathways | + | ||||
| 1 | Coagulation | |||||
| PID Pathway | [+] 1 PID Pathways | + | ||||
| 1 | Beta2 integrin cell surface interactions | |||||
| Reactome | [+] 6 Reactome Pathways | + | ||||
| 1 | Extrinsic Pathway of Fibrin Clot Formation | |||||
| 2 | Intrinsic Pathway of Fibrin Clot Formation | |||||
| 3 | Common Pathway of Fibrin Clot Formation | |||||
| 4 | Gamma-carboxylation of protein precursors | |||||
| 5 | Transport of gamma-carboxylated protein precursors from the endoplasmic reticulum to the Golgi apparatus | |||||
| 6 | Removal of aminoterminal propeptides from gamma-carboxylated proteins | |||||
| WikiPathways | [+] 5 WikiPathways | + | ||||
| 1 | Complement and Coagulation Cascades | |||||
| 2 | Human Complement System | |||||
| 3 | PTM: gamma carboxylation, hypusine formation and arylsulfatase activation | |||||
| 4 | Blood Clotting Cascade | |||||
| 5 | Formation of Fibrin Clot (Clotting Cascade) | |||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Pfizer. Product Development Pipeline. March 31 2009. | |||||
| REF 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||||
| REF 3 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6390). | |||||
| REF 4 | Nat Rev Drug Discov. 2013 Feb;12(2):87-90. | |||||
| REF 5 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2018 | |||||
| REF 6 | 2017 FDA drug approvals.Nat Rev Drug Discov. 2018 Feb;17(2):81-85. | |||||
| REF 7 | 2014 FDA drug approvals. Nat Rev Drug Discov. 2015 Feb;14(2):77-81. | |||||
| REF 8 | Drug information of Coagulation Factor IX, 2008. eduDrugs. | |||||
| REF 9 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6804). | |||||
| REF 10 | A comparison of danaparoid and lepirudin in heparin-induced thrombocytopenia. Thromb Haemost. 2001 Jun;85(6):950-7. | |||||
| REF 11 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7575). | |||||
| REF 12 | ClinicalTrials.gov (NCT01857622) Safety and Pharmacokinetics Study of DU-176b Administered to Non-valvular Atrial Fibrillation With Severe Renal Impairment. U.S. National Institutes of Health. | |||||
| REF 13 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. | |||||
| REF 14 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6388). | |||||
| REF 15 | 2011 FDA drug approvals. Nat Rev Drug Discov. 2012 Feb 1;11(2):91-4. | |||||
| REF 16 | FDA Accepts BPL's Amended BLA Submission for Coagadex (Coagulation Factor X, Human). BPL Bio Products Laboratory USA, Inc. | |||||
| REF 17 | ClinicalTrials.gov (NCT05878938) Open-label Safety Study in Adults and Adolescents With Haemophilia A With and Without FVIII Inhibitors Switching Directly From Emicizumab Prophylaxis to NNC0365-3769 (Mim8) Prophylaxis. U.S.National Institutes of Health. | |||||
| REF 18 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800031853) | |||||
| REF 19 | ClinicalTrials.gov (NCT00345618) Clinical Study Assessing SSR126517E Injections Once-weekly in Pulmonary Embolism Therapeutic Approach. U.S. National Institutes of Health. | |||||
| REF 20 | ClinicalTrials.gov (NCT00338897) Dose Ranging Study in Elective Total Hip Replacement Surgery. U.S. National Institutes of Health. | |||||
| REF 21 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800033781) | |||||
| REF 22 | The discovery and development of rivaroxaban, an oral, direct factor Xa inhibitor. Nat Rev Drug Discov. 2011 Jan;10(1):61-75. | |||||
| REF 23 | ClinicalTrials.gov (NCT00541320) Phase IIa Venous Thromboembolism (VTE) Prevention Study In Total Knee Replacement (TKR). U.S. National Institutes of Health. | |||||
| REF 24 | ClinicalTrials.gov (NCT00074828) New Oral Anticoagulant Therapy for the Prevention of Blood Clots Following Hip or Knee Replacement Surgery. U.S. National Institutes of Health. | |||||
| REF 25 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800024917) | |||||
| REF 26 | Clinical pipeline report, company report or official report of Roche | |||||
| REF 27 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800041083) | |||||
| REF 28 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800023539) | |||||
| REF 29 | ClinicalTrials.gov (NCT01954238) A Study to Access Safety, Tolerability, Pharmacokinetics(PK) and Pharmacodynamics(PD) of Orally Administered GCC-4401C in Healthy Volunteers. U.S. National Institutesof Health. | |||||
| REF 30 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800024856) | |||||
| REF 31 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800020651) | |||||
| REF 32 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800020726) | |||||
| REF 33 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800010432) | |||||
| REF 34 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800015497) | |||||
| REF 35 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800001281) | |||||
| REF 36 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2149). | |||||
| REF 37 | Juvenile hormone and octopamine in the regulation of division of labor in honey bee colonies. Horm Behav. 2002 Sep;42(2):222-31. | |||||
| REF 38 | DX-9065a Daiichi. Curr Opin Investig Drugs. 2003 Sep;4(9):1105-12. | |||||
| REF 39 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800004028) | |||||
| REF 40 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800024432) | |||||
| REF 41 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800003630) | |||||
| REF 42 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800027537) | |||||
| REF 43 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800012558) | |||||
| REF 44 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800012496) | |||||
| REF 45 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800022087) | |||||
| REF 46 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800014748) | |||||
| REF 47 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800010496) | |||||
| REF 48 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800009534) | |||||
| REF 49 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800004681) | |||||
| REF 50 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800022617) | |||||
| REF 51 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800004668) | |||||
| REF 52 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800012166) | |||||
| REF 53 | Effects of the oral, direct factor Xa inhibitor apixaban on routine coagulation assays and anti-FXa assays. J Thromb Haemost. 2014 Sep;12(9):1545-53. | |||||
| REF 54 | Company report (Portola) | |||||
| REF 55 | Haemophilia B: Christmas disease. Expert Opin Pharmacother. 2005 Aug;6(9):1517-24. | |||||
| REF 56 | Effect of factor X inhibition on coagulation activation and cytokine induction in human systemic inflammation. J Infect Dis. 2002 Nov 1;186(9):1270-6. | |||||
| REF 57 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 2359). | |||||
| REF 58 | Biochemistry and clinical pharmacology of new anticoagulant agents. Pathophysiol Haemost Thromb. 2002 Sep-Dec;32(5-6):218-24. | |||||
| REF 59 | Fondaparinux, a synthetic pentasaccharide: the first in a new class of antithrombotic agents - the selective factor Xa inhibitors. Cardiovasc Drug Rev. 2002 Winter;20(1):37-52. | |||||
| REF 60 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||||
| REF 61 | Serum zinc concentrations: contamination from laboratory equipment. JPEN J Parenter Enteral Nutr. 1979 May-Jun;3(3):179-81. | |||||
| REF 62 | Semuloparin for the prevention of venous thromboembolic events in cancer patients. Drugs Today (Barc). 2012 Jul;48(7):451-7. | |||||
| REF 63 | Venous thromboembolism in the patient with cancer: focus on burden of disease and benefits of thromboprophylaxis. Cancer. 2011 Apr 1;117(7):1334-49. | |||||
| REF 64 | Orally active thrombin inhibitors. Part 1: optimization of the P1-moiety. Bioorg Med Chem Lett. 2006 May 15;16(10):2641-7. | |||||
| REF 65 | Novel approaches to the treatment of thrombosis. Trends Pharmacol Sci. 2002 Jan;23(1):25-32. | |||||
| REF 66 | EP42675, a synthetic parenteral dual-action anticoagulant: pharmacokinetics, pharmacodynamics, and absence of interactions with antiplatelet drugs. J Thromb Haemost. 2014 Jan;12(1):24-33. | |||||
| REF 67 | Company report (Sanofi) (drug: FY2008) | |||||
| REF 68 | The pharmacokinetics of darexaban are not affected to a clinically relevant degree by rifampicin, a strong inducer of P-glycoprotein and CYP3A4. Br J Clin Pharmacol. 2013 Feb;75(2):440-9. | |||||
| REF 69 | Pharma & Vaccines. Product Development Pipeline. April 29 2009. | |||||
| REF 70 | Non-hemostatic activity of coagulation factor Xa: potential implications for various diseases. Curr Opin Pharmacol. 2001 Apr;1(2):169-75. | |||||
| REF 71 | Description of the chemical and pharmacological characteristics of a new hemisynthetic ultra-low-molecular-weight heparin, AVE5026. J Thromb Haemost. 2009 Jul;7(7):1143-51. | |||||
| REF 72 | AVE5026, a new hemisynthetic ultra-low-molecular-weight heparin for the prevention of venous thromboembolism in patients after total knee replaceme... J Thromb Haemost. 2009 Apr;7(4):566-72. | |||||
| REF 73 | DX-9065a inhibition of factor Xa and the prothrombinase complex: mechanism of inhibition and comparison with therapeutic heparins. Thromb Haemost. 2003 Jan;89(1):112-21. | |||||
| REF 74 | Nilotinib: a novel, selective tyrosine kinase inhibitor. Semin Oncol. 2011 Apr;38 Suppl 1:S3-9. | |||||
| REF 75 | Synthesis and evaluation of 1-arylsulfonyl-3-piperazinone derivatives as a factor Xa inhibitor II. Substituent effect on biological activities. Chem Pharm Bull (Tokyo). 2002 Sep;50(9):1187-94. | |||||
| REF 76 | Clinical pipeline report, company report or official report of Takeda (2009). | |||||
| REF 77 | The design and synthesis of noncovalent factor Xa inhibitors. Curr Top Med Chem. 2001 Jun;1(2):137-49. | |||||
| REF 78 | Expression of biological activity of draculin, the anticoagulant factor from vampire bat saliva, is strictly dependent on the appropriate glycosylation of the native molecule. Biochim Biophys Acta. 1998 Oct 23;1425(2):291-9. | |||||
| REF 79 | Effects of tannins from Geum japonicum on the catalytic activity of thrombin and factor Xa of blood coagulation cascade. J Nat Prod. 1998 Nov;61(11):1356-60. | |||||
| REF 80 | 3,6-disubstituted coumarins as mechanism-based inhibitors of thrombin and factor Xa. J Med Chem. 2005 Dec 1;48(24):7592-603. | |||||
| REF 81 | Design of selective phenylglycine amide tissue factor/factor VIIa inhibitors. Bioorg Med Chem Lett. 2005 Feb 1;15(3):817-22. | |||||
| REF 82 | Aroylguanidine-based factor Xa inhibitors: the discovery of BMS-344577. Bioorg Med Chem Lett. 2009 Dec 15;19(24):6882-9. | |||||
| REF 83 | Discovery of 1-(4-methoxyphenyl)-7-oxo-6-(4-(2-oxopiperidin-1-yl)phenyl)-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxamide (apixaban, BMS... J Med Chem. 2007 Nov 1;50(22):5339-56. | |||||
| REF 84 | Effects of ZK-807834, a novel inhibitor of factor Xa, on arterial and venous thrombosis in rabbits. J Cardiovasc Pharmacol. 2000 May;35(5):796-805. | |||||
| REF 85 | Effect of vascular injury on inhibition of venous thrombosis with ZK-807834, a direct inhibitor of factor Xa. J Thromb Haemost. 2003 Sep;1(9):1955-8. | |||||
| REF 86 | Novel 3-carboxamide-coumarins as potent and selective FXIIa inhibitors. J Med Chem. 2008 Jun 12;51(11):3077-80. | |||||
| REF 87 | How many drug targets are there Nat Rev Drug Discov. 2006 Dec;5(12):993-6. | |||||
| REF 88 | Synthesis and evaluation of 1-arylsulfonyl-3-piperazinone derivatives as factor Xa inhibitor. Chem Pharm Bull (Tokyo). 2001 Oct;49(10):1237-44. | |||||
| REF 89 | Role of tissue factor pathway inhibitor in the regulation of tissue factor-dependent blood coagulation. Cardiovasc Drug Rev. 2002 Winter;20(1):67-80. | |||||
| REF 90 | Inhibitors of proteases and amide hydrolases that employ an alpha-ketoheterocycle as a key enabling functionality. Bioorg Med Chem. 2008 Feb 15;16(4):1562-95. | |||||
| REF 91 | Pharmacological intervention at disparate sites in the coagulation cascade: comparison of anti-thrombotic efficacy vs bleeding propensity in a rat model of acute arterial thrombosis. J Thromb Thrombolysis. 2002 Oct;14(2):113-21. | |||||
| REF 92 | Synthesis and biological activity of novel 1,4-diazepane derivatives as factor Xa inhibitor with potent anticoagulant and antithrombotic activity. Bioorg Med Chem. 2004 May 1;12(9):2179-91. | |||||
| REF 93 | Thiophene-anthranilamides as highly potent and orally available factor Xa inhibitors. J Med Chem. 2007 Jun 28;50(13):2967-80. | |||||
| REF 94 | Discovery of the novel antithrombotic agent 5-chloro-N-({(5S)-2-oxo-3- [4-(3-oxomorpholin-4-yl)phenyl]-1,3-oxazolidin-5-yl}methyl)thiophene- 2-carb... J Med Chem. 2005 Sep 22;48(19):5900-8. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

